
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Avidin inhibits PHA-induced human peripheral blood mononuclear cell proliferation
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i1.1264 Med J Indones. 2016;25:19–24
Received: July 14, 2015
Accepted: March 09, 2016
Author affiliation:
1 Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Medical Biology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Center of Hypoxia and Oxidative Stress Studies, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Cicia Firakania
E-mail: ciciafirakania@gmail.com
Background
Cell proliferation occurs not only in normal but also in cancer cells. Most of cell proliferation inhibition can be done by inhibiting the DNA synthesis, notably by intervening the formation of purine or pyrimidine. In purine de novo synthesis, it was assumed that biotin plays a role as a coenzyme in carboxylation reaction, one of the pivotal steps in the purine de novo pathways. The aim of this study was to see the avidin potency to bind biotin and inhibit mitosis.
Methods
Peripheral blood mononuclear cell (PBMC) was cultured in RPMI-1640 medium and stimulated by phytohemagglutinin (PHA) in the presence or absence of interleukin-2 (IL-2), with or without avidin. The effect of avidin addition was observed at 24, 48, and 72 hours for cell proliferation, viability, and cell cycle. Statistical analysis was done by one-way ANOVA.
Results
Avidin inhibited cell proliferation and viability in culture under stimulation by PHA with and without IL-2. Cell cycle analysis showed that avidin arrested the progression of PBMC after 72 hours of culture. Most cells were found in G0/G1 phase.
Conclusion
Inhibition of biotin utilization by avidin binding can halt cell proliferation.
Keywords
avidin, biotin, cell proliferation, purine de novo synthesis
Purine is an essential compound in nucleotides and functions as building block of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).1 Nucleotides also have a role as a second messenger, such as guanosine triphospate (GTP) and 3’-5’-cyclic adenosine monophospate (cAMP), and as a coenzyme such as adenine dinucleotide (NAD) and coenzyme A.2 The inhibition of purine synthesis is important to stop cell proliferation. In cancer, cell proliferation occurs continuously and uncontrolled.3 An attempt to halt cell proliferation is the cornerstone of cancer therapy. Cell proliferation can be inhibited in various ways, most of them by intervention of purine synthesis.4
There are two pathways in purine synthesis, de novo synthesis pathway and salvage pathway. In de novo synthesis, purine is synthesized directly from precursors, i.e. sugar, amino acids, and bicarbonate. In salvage pathway, the purine or nucleotide released from hydrolytic degradation of nucleic acid is reused or recycled.5 In de novo synthesis pathway, there are ten enzymatic reactions, three of the reactions need the vitamin as a coenzyme. Reaction in the third and the ninth step needs the folic acid, whereas reaction in the sixth step, a carboxylation reaction, commonly needs the biotin.6
To date, intervention of the formylation step is used as a therapeutical approach, by using the folate analogues. It is interesting to note that the sixth step which needs biotin is practically not explored at all. Avidin is a well known protein due to its high affinity to biotin (Kd=10-15M).7 Avidin-biotin interaction is considered the strongest non-covalent interaction among ligand-protein interaction in nature.8 So far, utilization of the interaction is limited to in vitro assay and other applications such as enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and western blot.9 We supposed, based on the high affinity of biotin-avidin interaction, this protein could arrest cell proliferation. In this study, we evaluated the role of biotin on DNA synthesis, cell proliferation, cell cycle by using human peripheral blood mononuclear cell (PBMC) stimulated to mitosis with phytohemagglutinin (PHA). The cells were then harvested periodically after the first stimulation.
METHODS
This was an experimental study conducted at the Department of Biochemistry and Molecular Biology, Department of Medical Biology, and Department of Clinical Pathology, Faculty of Medicine, Universitas Indonesia, Jakarta, from March 2014 to March 2015.
The protocol of the study has been approved by Medical Ethics Committee of Faculty of Medicine, Universitas Indonesia (No. 318/ H2.F1/ETIK/2014). Signed informed consent was obtained from three volunteers. PBMC was obtained from the buffy coats of healthy donors. Heparinized human peripheral blood (6 mL) was obtained from normal healthy volunteers. Peripheral blood was isolated by gradient density centrifugation with Ficoll Paque PLUS (GE HealthcareTM). Cells in the interface layers were removed and washed by phosphate buffered saline (PBS), then resuspended in RPMI-1640 (LonzaTM) medium which is supplemented with 10% inactive FBS (GibcoTM), 100 U/mL penicillin (GibcoTM), and 100 μg/mL streptomycin (GibcoTM). Cells were cultured in triplicate with density of 0.5x106 cell/mL and stimulated by addition of 10 U/mL IL-2 (PeprotechTM) and 1% PHA (GibcoTM). BrdU incorporation and the reduction of tetrazolium salt were used to determine the cells proliferation and viability. Cells were harvested at 24, 48, and 72 hours after the addition of avidin.
The proliferation of PBMC was done using commercial Kit Cell Proliferation ELISA BrdU (colorimetric) (RocheTM) following the procedure described in the user’s manual. The viability measurement of PBMC was carried out using the commercial kit Proliferation Reagent WST-1 (RocheTM). Data were expressed as absorbance (mean±SD) of at least triplicate experiments. Cells (1x106) were harvested, washed with PBS, fixed with 70% ethanol, treated with 100 μg/mL RNase A (Sigma- AldrichTM), and stained with 50 μg/mL propidium iodide (Sigma-AldrichTM). After 30 minutes incubation at room temperature, the distribution of DNA content was measured with a FACS Calibur (Beckton Dickinson Immunocytometry SystemsTM) flow cytometer.
Data analysis was done by using SPSS version 19. Normality of data was tested by Kolmogorov- Smirnov test. Comparison among groups was done by one-way ANOVA.
RESULTS
Effect of avidin on proliferation and cell viability in PHA-stimulated human PBMC
To assess the effect of avidin in inhibiting cell proliferation and viability, PBMC were stimulated by PHA (10 μg/mL) and PHA (10 μg/mL) plus IL-2 (10 μ/mL). At 24, 48, and 72 hours intervals, the wells seeded at 5x104 cells were harvested and analyzed by BrdU ELISA proliferation assay which reveal cell in the S phase of the cell cycle. The cells stimulated with PHA proliferated significantly (p<0.05) higher compared to non-stimulated cells over 48 hours culture. However, the cells number after 72 hours was decreased (p<0.05). The proliferation of PBMC was inhibited by avidin. Significant inhibition was observed at 24 hours (p<0.05), 48 hours (p<0.05), and 72 hours (p<0.05) (Figure 1).
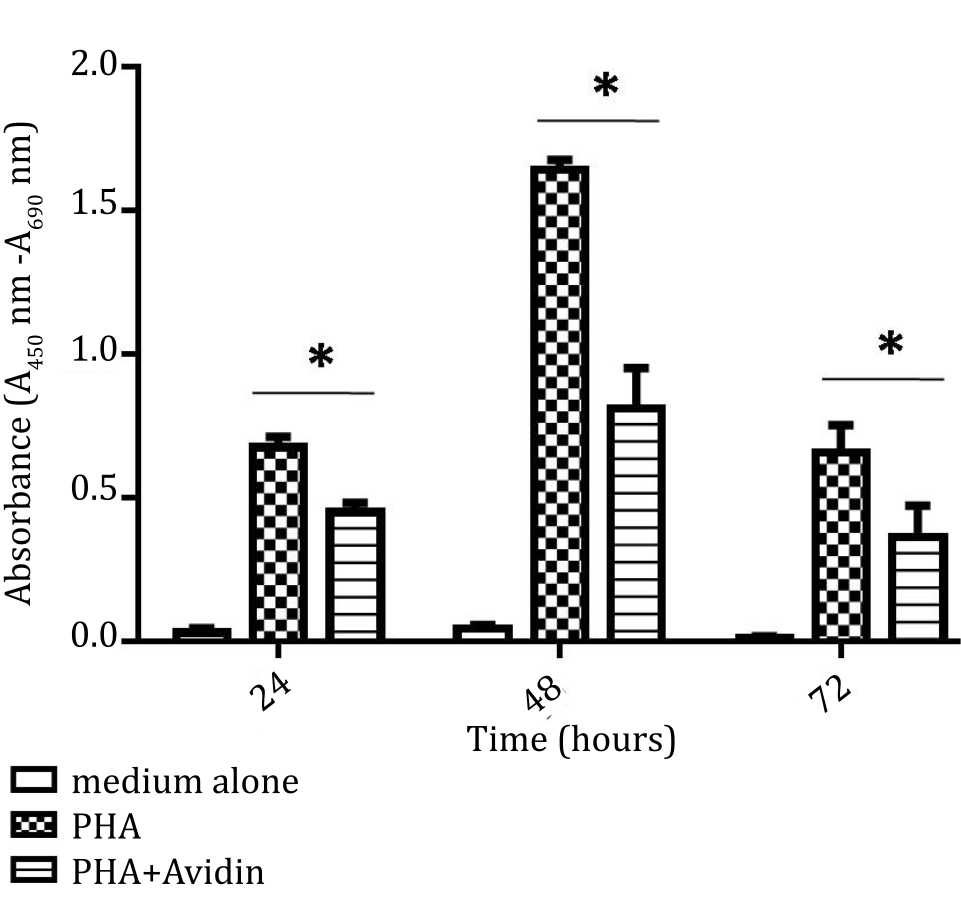
Figure 1. Effect of purine synthesis inhibition by avidin on cell proliferation under stimulation with phytohemagglutinin (PHA). The absorbance based on BrdU incorporation was used as indicator of proliferation and evaluated at 24, 48, and 72 hours (*= p<0.05)
There was a significant increase of cell proliferation after stimulation by PHA and IL-2 compared to non-stimulated cells over 48 hours culture. The proliferation of PBMC was significantly inhibited by addition of avidin. As shown in Figure 1, proliferation of PBMC was also decreased at 72 hours (Figure 2).
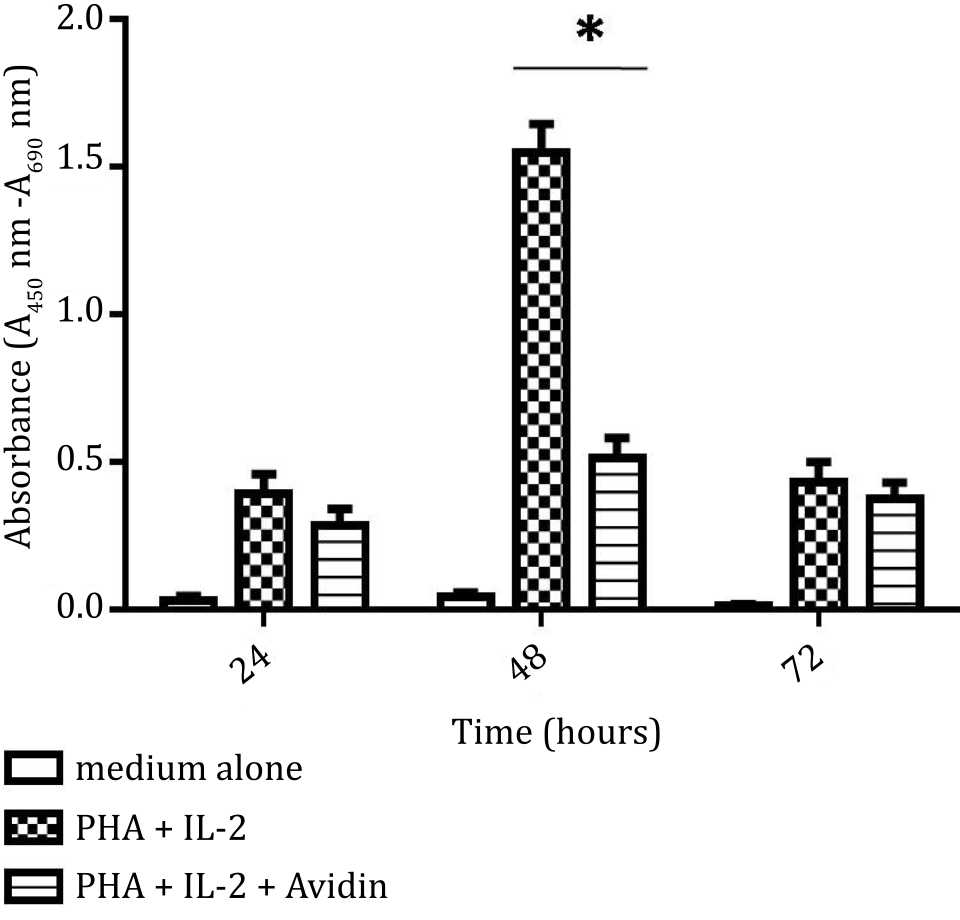
Figure 2. Effect of purine synthesis inhibition by avidin on cell proliferation under stimulation with phytohemagglutinin (PHA) and interleukine 2 (IL-2). The absorbance based on BrdU incorporation was used as indicator of proliferation and evaluated at 24, 48, and 72 hours (*= p<0.05)
To assess the cell viability, PBMC was stimulated with PHA and in the medium containing PHA and IL-2. The effect of the addition of avidin was observed at 24, 48, and 72 hours for cell viability analysis. There was a significant increase of cell viability after stimulation by PHA compared to non-stimulated cells over 72 hours culture. Significant inhibition was observed at 72 hours (p<0.05) (Figure 3).
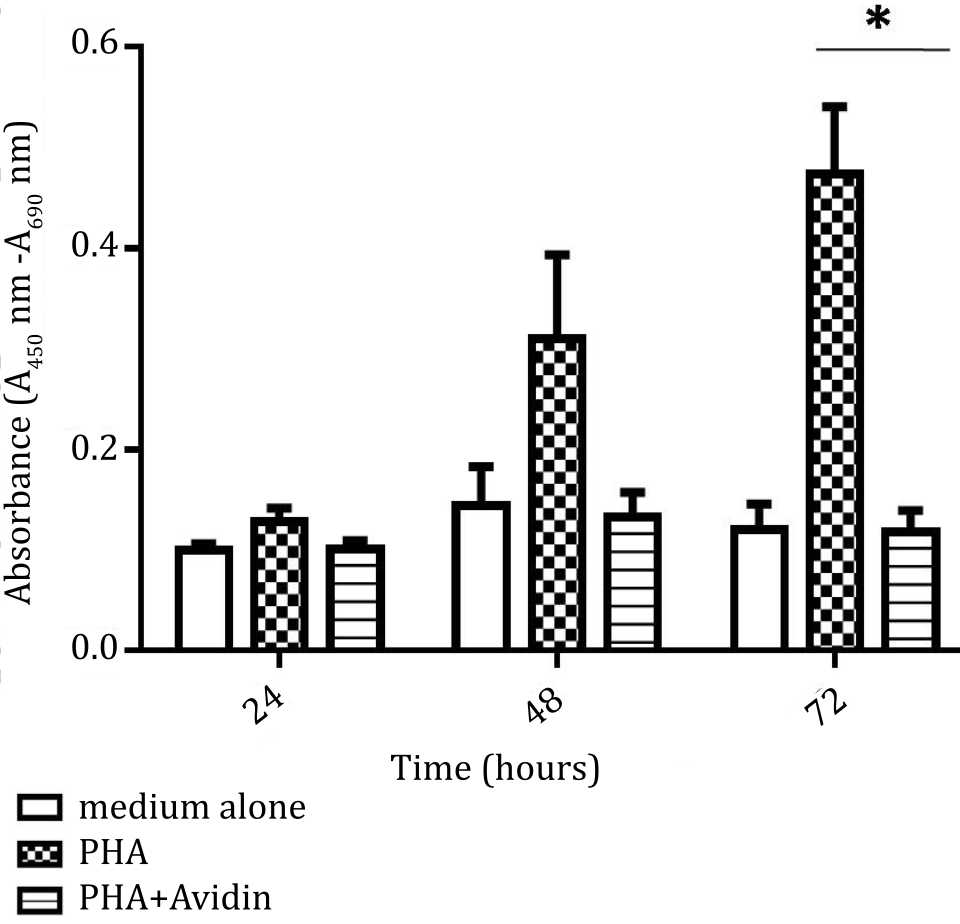
Figure 3. Effect of purine synthesis inhibition by avidin on cell viability under stimulation with phytohemagglutinin (PHA). The absorbance based on the reduction of tetrazolium salt was used as indicator of cell viability, and evaluated at 24, 48, and 72 hours (*= p<0.05)
The cell viability was significantly increased in cell stimulated with PHA and IL-2, compared to unstimulated cells over 72 hours cultures (p<0.05). Addition of avidin was associated with significant decrease of cell viability at 24 and 72 hours (p<0.05) (Figure 4).
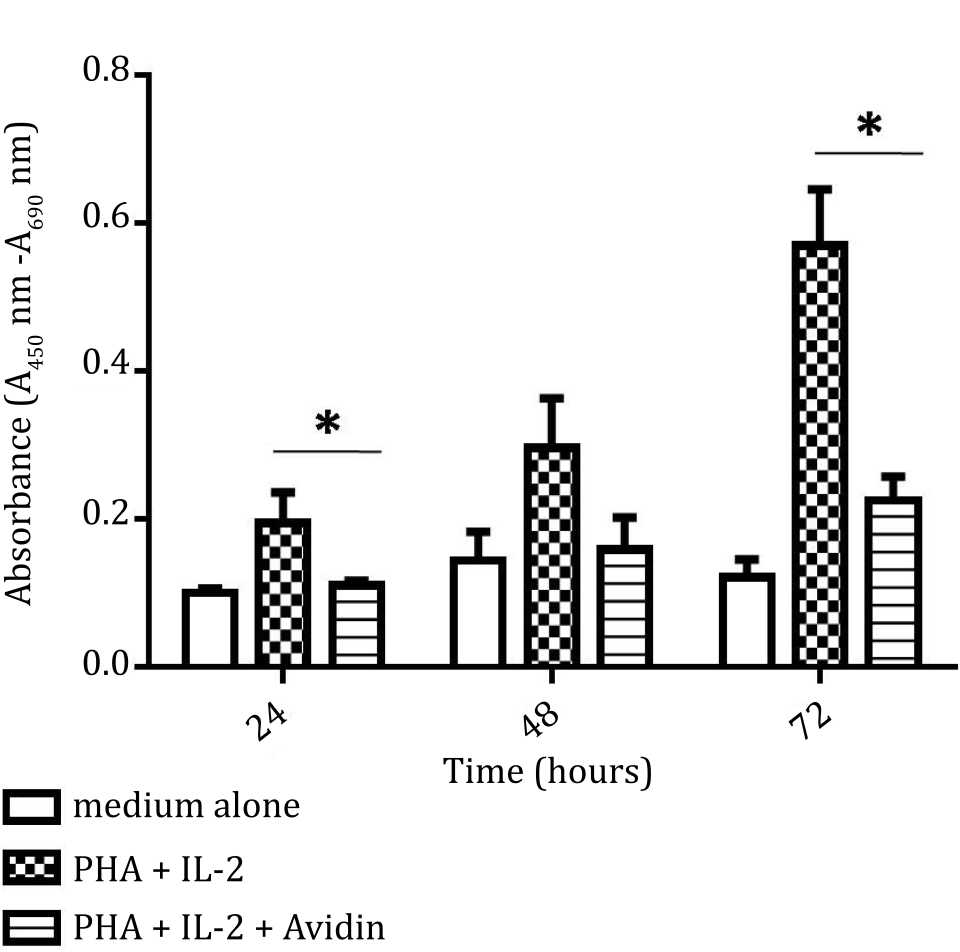
Figure 4. Effect of purine synthesis inhibition by avidin on cell viability under stimulation with phytohemagglutinin (PHA) and interleukin 2 (IL-2). The absorbance of cell viability was measured based on tetrazolium salt reduction and evaluated at 24, 48, and 72 hours (*= p<0.05)
Distribution of PBMC stimulated by PHA with and without avidin at various phases of cell cycle
It can be seen that PBMC in control group (Figure 5A) and after stimulation with PHA (Figure 5B) were in G1 phase (M1). However, the cells were already started to DNA synthesis phase, and in the end the population entered mitosis phase. Most of PHA and avidin stimulated-PBMC were also in G1 phase (M1). Addition of avidin was associated with lower number of cells undergoing DNA synthesis compared to those treated with PHA alone (Figure 5C).
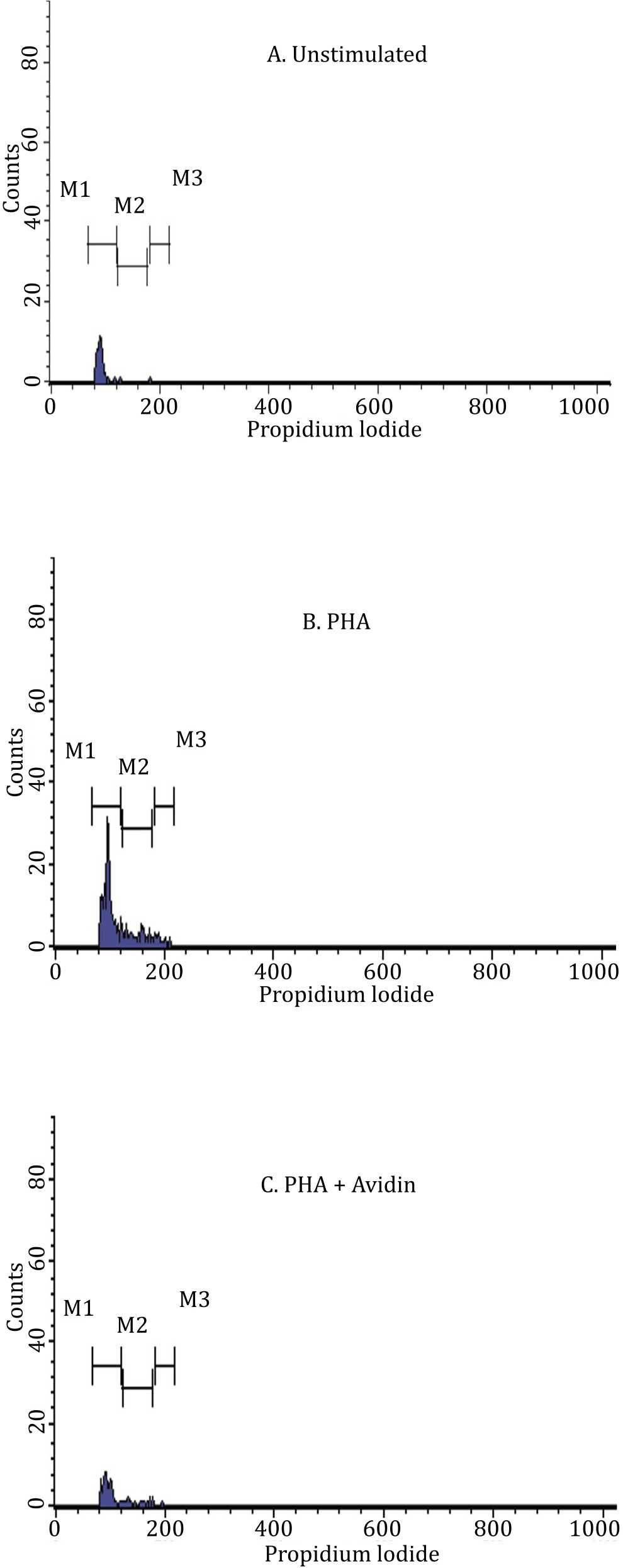
Figure 5. Antiproliferative effect of avidin in the mitosis of PBMC. PBMC was stained by propidium iodide. PBMC was cultured in medium alone (panel A), stimulated by PHA (panel B), and stimulated with PHA with addition of avidin (panel C). Analysis of viable cell was done with FACS Calibur (Beckton Dickinson Immunocytometry Systems) and evaluated after 72 hours of incubation
Figure 6 shows that PBMC seeded in medium alone (control) entered the G0/G1 phase 97.28%, S phase 1.36%, and G2/M 0.68%. The PHAstimulated PBMC, entered the G0/G1 phase 71.13%, S phase 21.48%, and G2/M 5.31%. The addition of avidin in PHA-stimulated PBMC, entered the G0/G1 phase 80.53%, S phase 17.70%, and G2/M 0.88%.
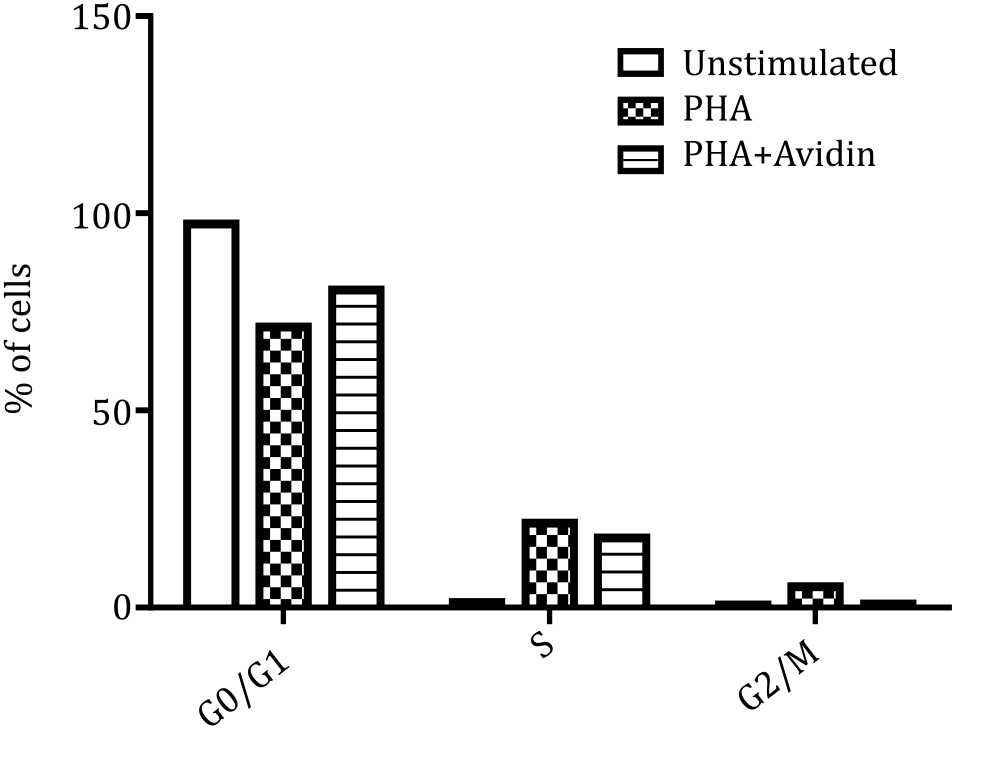
Figure 6. Distribution of PBMC in cell cycle phase in control group, PHA-stimulated group and influence of avidin on each phases
DISCUSSION
Peripheral blood mononuclear cell is used as model in various studies of cell proliferation as well as to evaluate the effects of various treatments on this process.10 The majority of the cells are T lymphocytes and this cell can easily be stimulated to mitosis by adding phytohemagglutinin (PHA).10,11 The advantage of this model is that the mitosis can be started anytime and the cells proliferate simultaneously and synchronically. Therefore, the cells can be harvested at any time as needed and the responding cells are practically found at the same stage of cell cycle.
Purine is an indispensable part of DNA.4 As mentioned previously, purine is made in two ways, the de novo synthesis and the salvage pathways. The differences of both pathways are laid on the fact that in the de novo synthesis, purine is synthesized directly from precursors and requires several cofactors.5 There are three steps in which two different groups are needed to complete the reaction, the formyl, and carboxyl groups.1
Intervention of formylation process will result in inhibition of purine synthesis. This intervention can be obtained by using folic acid analogue.12 It was proven in microbes (Saccharomyces cerevisiae and also L. arabinosus), that purine synthesis needs biotin for the CO2 fixation.13 The phenomenon is also observed in the pigeon liver homogenates.14 Zerega et al15 have proven that avidin, a biotin binder, inhibited chick chondrocytes proliferation in vitro. These authors explained that biotin deprivation interfered the activity of fatty acid metabolism, i.e. acetyl-CoA carboxylase, pyruvate carboxylase, and the β-sub unit of the propionyl-CoA carboxylase. These enzymes play a pivotal role in the terminal stage of differentiation. In higher organism, it was observed in rats that biotin was needed in the fixation of CO2 into purines.16
To date, there was no report about the effect of intervention of carboxylation process by addition of biotin binder which makes this cofactor not available any longer. It is well known that avidin binds the biotin very strongly with the Kd of 10-15.7 Once bound, it is practically impossible to dissociate avidin-biotin complex. If it happened, the bounded biotin cannot be used anymore. In this study, BrdU (analogue of thymidine) incorporation was used as indicator DNA synthesis,17 whereas the formazan formed from water soluble tetrazolium-1 Salts (WST-1) was used as indicator of cell proliferation and viability.18
Decreases DNA synthesis and viability can be seen by low BrdU incorporation and the low optical density value of the WST-1 assay. In this obeservation, we found that BrdU incorporation and the reduction of WST-1 tetrazolium salt were significantly lower in stimulated PBMC treated by avidin. Occurence of proliferation arrest was also demonstrated by the flowcytometry analysis. As the phenomenon is found only in the PHA-stimulated PBMC treated by avidin, it is suggested that the important part of biotin found previously in the medium are bound by the avidin and therefore biotin are not available anymore for the cell. Flowcytometry analysis also indicated that proliferation of avidin treated cells was halted in the G0/G1 phase. It means that the cell cannot enter the S phase in which the DNA synthesis occurs.
To date, the inhibition of DNA synthesis i.e. in cancer treatment is done by using purine or pyrimidine analogues or by intervening the purine synthesis using the folate analogue. Our observations indicate that disturbing the biotin availability, in this case by using avidin, can halt DNA synthesis and decrease cell viability. Unlike purine or pyrimidine analogues, avidin binds the biotin very firmly. In the principal, using a specific binding protein for inhibition of DNA synthesis is rather difficult to use in the whole intact animals. However, there are some possibilities to be considered, for instance by using biotin analogue in DNA synthesis. However utilization of a biotin analogue, which is a xenobiotic, is not without problems. On the other hand, utilization of intact avidin, which is a foreign protein will provoke an immune response. For this reason, a possible approach is to construct a small peptide which can bind the biotin. The peptide can be constructed by studying the binding site of avidin.
In conclusion, our study indicated that intervention of biotin availability by avidin could reduce or even halt cell proliferation and viability. The analysis of cell cycle showed that addition of avidin inhibit the progression of cell from the G0/ G1 to S phase.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The authors are grateful to receive research study grant from Universitas Indonesia (Hibah Penelitian Unggulan Perguruan Tinggi Universitas Indonesia). Academic years 2014. No: 0958/H2.R12/HKP.05.00/2014.
REFERENCES
- Murray RK, Bender DA, Botham KM, Kennely PJ, Rodwell VW, Weil PA. Harper’s Illustrated Biochemistry. 28th ed. New York; Mc Graw Hill: 2009. p. 289–90.
- Fang Y, French J, Zhao H, Benkovic S. G-protein-coupled receptor regulation of de novo purine biosynthesis: a novel druggable mechanism. Biotechnol Genet Eng Rev. 2013;29:31–48.
- Gérard C, Goldbeter A. The balance between cell cycle arrest and cell proliferation: control by the extracellular matrix and by contact inhibition. Interface Focus. 2014;4(3):20130075
- Adam T. Purine de novo synthesis–mechanisms and clinical implications. Klin Biochem Metab. 2005;13(34):177–81.
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th ed. New York; WH Freeman: 2002. p. 1030–31.
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 5th ed. New York; WH Freeman and Company: 2008. p. 882–84.
- Livnah O, Bayert EA, Wilchek M, Sussman JL. Threedimensional structures of avidin and the avidin-biotin complex. Proc Natl Acad Sci U S A. 1993;90(11):5076–80.
- Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, Uhlén M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis. 2005;26(3):501–10.
- Beckett D, Kovaleva E, Schatz PJ. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 1999;8(4):921–9.
- Chilson OP, Boylston AW, Crumpton MJ. Phaseolus vulgaris phytohaemagglutinin (PHA) binds to the human T lymphocyte antigen receptor. EMBO J. 1984;3(13):3239–45.
- Quéméneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, Genestier L. Differential control of cell cycle, proliferation, and survival of primary T Lymphocytes by purine and pyrimidine nucleotides. J Immunol. 2003;170(10):4986–95.
- Dervieux T, Brenner TL, Hon YY, Zhou Y, Hancock ML, Sandlund JT, et al. de novo purine synthesis inhibition and antileukemic effects of mercaptopurine alone or in combination with methotrexate in vivo. Blood. 2002;100(4):1240–7.
- Wahba AJ, Shive W. A role of aspartic acid in purine biosynthesis. J Biol Chem. 1954:211(1):155–61.
- Levenberg B, Buchanan JM. Biosynthesis of the purines. XII. Structure, enzymatic synthesis, and metabolism of 5-amino-imidazole ribotide. J Biol Chem. 1957;224(2):1005–18.
- Zerega B, Camardella L, Cermelli S, Sala R, Cancedda R, Descalzi Cancedda F. Avidin expression during chick chondrocyte and myoblast development in vitro and in vivo: regulation of cell proliferation. J Cell Sci. 2001;114(Pt8):1473–82.
- McLeod PR, Lardy HA. Metabolic function of biotin; the fixation of carbon dioxide by normal and biotindeficient rats. J Biol Chem. 1949;179(2):733–41.
- Messele T, Roos MTL, Hamann D, Koot M, Fontanet AL, Miedema F, et al. Nonradioactive techniques for measurement of in vitro t-cell proliferation: alternatives to the [3h]thymidine incorporation assay. Clin Diagn Lab Immunol. 2000;7(4):687–92.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–52.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id