
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Human plasma glial fibrillary acidic protein and heat shock protein 27 concentrations in acute moderate-intensity aerobic exercise with different duration
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i2.1267 Med J Indones. 2016;25:112–7
Received: July 31, 2015
Accepted: April 19, 2016
Author affiliation:
1 Biomedical Program, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Physiology, Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Indonesia
3 Department of Physiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Sophie Yolanda
E-mail: sophiehijau@gmail.com
Background
Glial fibrillary acidic protein (GFAP) and heat shock protein -27 (HSP27) plasma can be used as the parameters of exercise-induced astrocyte reactivity. The American College of Sports Medicine (ACSM) recommends an exercise of 30 minutes or 10 minutes duration (each performing bout accumulated toward 30 minutes). The aim of this study was to compare GFAP and HSP27 plasma concentrations in young adults undergoing acute moderate-intensity aerobic exercise of different durations (10 minutes vs 30 minutes).
Methods
An experimental study with pre-post design was conducted on 22 participants assigned to either 10 minutes or 30 minutes duration of single bout exercise. Blood sampling was performed before and after the exercise. GFAP and HSP27 plasma levels were measured with ELISA methods. Plasma GFAP and HSP27 levels before and after exercise were analyzed using paired t-test, while GFAP and HSP27 levels after exercise between the two groups were processed using unpaired t-test.
Results
Plasma GFAP concentration decreased significantly (0,45 ng/mL) after 30 minutes of aerobic exercise (p<0.05). Plasma HSP27 concentration decreased significantly (1,71 ng/mL) after 10 minutes of aerobic exercise (p<0.05). No significant difference in plasma GFAP and HSP27 concentrations between 10 minutes (GFAP=0.49 ng/mL; HSP27= 2.09 ng/mL) and 30 minutes duration of exercise (GFAP=0.45 ng/mL; HSP27=1,71 ng/mL).
Conclusion
Acute moderate-intensity aerobic exercise with 10- and 30-minutes duration reduces the reactivity of astrocytes indication the increase of the synapse plasticity. The decrease in GFAP concentration occurred after 30 minutes of exercise and the decrease in HSP27 occurred after 10 minutes of exercise. These results showed that the body responds differently to different treatment duration in order to obtain the same effect on the body.
Keywords
aerobic exercise, astrocytes reactivity, GFAP, HSP27, synaptic plasticity
Neurodegenerative diseases such as stroke, dementia, Parkinson’s disease, have become a public health problems. The incidence of neurodegenerative diseases is increasing from year to year.1 This increasing incidence is partly caused by unhealthy lifestyle, such as lack of exercise, smoking, and unhealthy diet.1 Physical exercise represents one way to prevent neurodegenerative diseases.
Many studies proved that physical exercise has a positive impact on human and animal brain.2,3 Physical exercise increases the plasticity of synapses by affecting the neurons and glial cells. The recommendations of American College Sports Medicine (ACSM) exercise for healthy adult aged 18 to 65 is moderate-intensity aerobic physical activity for 30 minutes five days per week or 10 minutes each performing bout (accumulated toward 30 minutes).4
Astrocytes are the main glial cells that play an important role in synaptic plasticity. Its important role in synaptic plasticity is by supporting the nerve cells in several ways such as efficiency of synaptic connections, maintenance of neurotransmitter homeostasis, formation of the blood brain barrier, protection of central nervous system damage, and provision of nutrition (metabolic functions).5
Glial fibrillary acidic protein (GFAP) is an intermediate filaments (IF) protein of astrocytes and have been used as a classical marker for studying astrocytes in healthy and pathological state.5 Astrocytes become reactive to pathological conditions and this reactivity is marked by upregulation of GFAP.5 Studies showed that physical exercise affects GFAP.2 Heat shock protein 27 (HSP27) is one of the factors induced by physical exercise to affect GFAP.6-8 Studies showed that HSP27 interacts with GFAP and serves as chaperones (preventing of protein aggregation).8 Release of HSP27 to plasma indicates that cells are in stress.6,7 HSP27 peaks in plasma approximately one hour after stress.7
Besides physical exercise, other factors affecting GFAP are brain development, aging, heat stress, and neurodegenerative diseases.5-7 Brain development and aging will trigger molecules that will modulate GFAP, such as ciliary neurotrophic factor (CNTF) and nuclear factor kappa B (NFκB).5
Heat stress and neurodegenerative diseases will trigger molecules that will modulate HSP27, such as glucocorticoid.6,7
The studies on the effects of physical exercise on plasma GFAP levels in humans is unknown, except in subjects with neurological disease or normal subjects without physical exercise.9,10 GFAP levels in blood of healthy people ranges from non-detectable to <0.76 μg/L.10 The low level of GFAP in normal human plasma showed normal astrocytes. Release of GFAP to plasma indicates reactive astrocytes.5,9,10 In case of head injury, the release of GFAP to plasma peaks after 24 hours.10 Studies on the effects of physical exercise on GFAP are mainly conducted in animals2 while human studies are very limited. In addition, studies on the relationship of HSP-27 and GFAP under the influence of exercise are very limited.
The aim of this study was to evaluate plasma GFAP and HSP27 concentration in acute moderateintensity aerobic exercise of different durations (10 or 30 minutes). This study is expected to be a guide for selecting the duration of aerobic physical exercise for people who are new to the practice as well as those with high risk for neurodegenerative disease.
METHODS
Participants were 22 young healthy male adults aged 20.86±0.88 years, were recruited from Faculty of Medicine Universitas Atmajaya, Jakarta, Indonesia. They were untrained men, with normal anthropometric measurements and not subjected to exam-related stress. Participants were evaluated for their health/fitness using an examination form of physical fitness/health from Indonesian Ministry of Health and questionnaire of health/fitness from American Heart Association (AHA) or ACSM to ensure that they could safely complete the study.4,11 This study used the guidelines of the ACSM for the duration and intensity of aerobic exercise recommended for the age 18–65 years.4
The protocol of this study has been approved by the Health Research Ethics Committee Faculty of Medicine, Universitas Indonesia No. 883/UN2.F1/ETIK/2014. All participants signed informed consent.
Age level was determined through selfreporting. Resting heart rate and blood pressure were measured using the electronic sphygmomanometer (Omron, Europe). Height and weight were measured, from which body mass index were calculated. Waist circumference was measured using measuring tape.
Exercise intervention
Participants were divided into two treatment groups, the first group (11 people) performed exercise in moderate-intensity {64–74% maximum heart rate (-HRmax)} for 10 minutes and the other group (11 people) performed exercise with the same intensity for 30 minutes. The exercise was performed in a single bout exercise. Participants experienced three phases, i.e pre-exercise, exercise, and post-exercise phases.
In the pre-exercise phase, participants were measured for heart rate and blood pressure. Then, a heart rate monitor was placed on the chest to record heart rate per minute (Polar Electro, Finland). The exercise uses a static bicycle (Monark, Sweden) with the exercise protocol consisting of a two-minutes warmup period, a 10- or 30-minutes core exercise period, and a five-minutes cool-down period. The speed of cycling was set using a metronome at 100 times/min and the workload was set until participants reached their target HR between 64–74% of their HRmax to achieve moderate intensity. HRmax was calculated using the 220-age formula.4 In the post-exercise phase, participants were measured for heart rate and blood pressure again.
Blood sampling and examination
Venous bloods sampling was taken before and after cycling exercise from median cubital vein. Venous blood was stored in ethylenediaminetetraacetic acid (EDTA) tubes. The blood was centrifuged at 1.000 g and 4°C for 15 minutes, and the supernatant were stored at -80 °C until analysis.
Plasma GFAP and HSP27 concentrations were analyzed using GFAP ELISA Kit (SEA068Hu, USCNK Cloud Clone Corp, USA) and HSP27 ELISA Kit (ADI-EKS-500, Enzo Life Science, USA). The minimum detectable level of GFAP ELISA Kit is 0.059 ng/mL, while the minimum detectable level of HSP27 ELISA kit is 0.39 ng/mL.
Statistical analysis
Statistical analysis was performed using SPSS 16. Statistical significance was set at p<0.05. Shapiro-Wilk test showed all data were normally distributed, except for age. Demographic data was tested with unpaired t-test, except the age data was tested with Mann-Whitney test. Workout heart rate, GFAP and HSP27 levels before and after moderate-intensity aerobic exercise of 10- and 30-minutes duration were tested with paired t-test. Workout HR, GFAP levels and HSP27 levels after 10- and 30-minutes aerobic exercise were compared with unpaired t-test.
RESULTS
Demographic variables
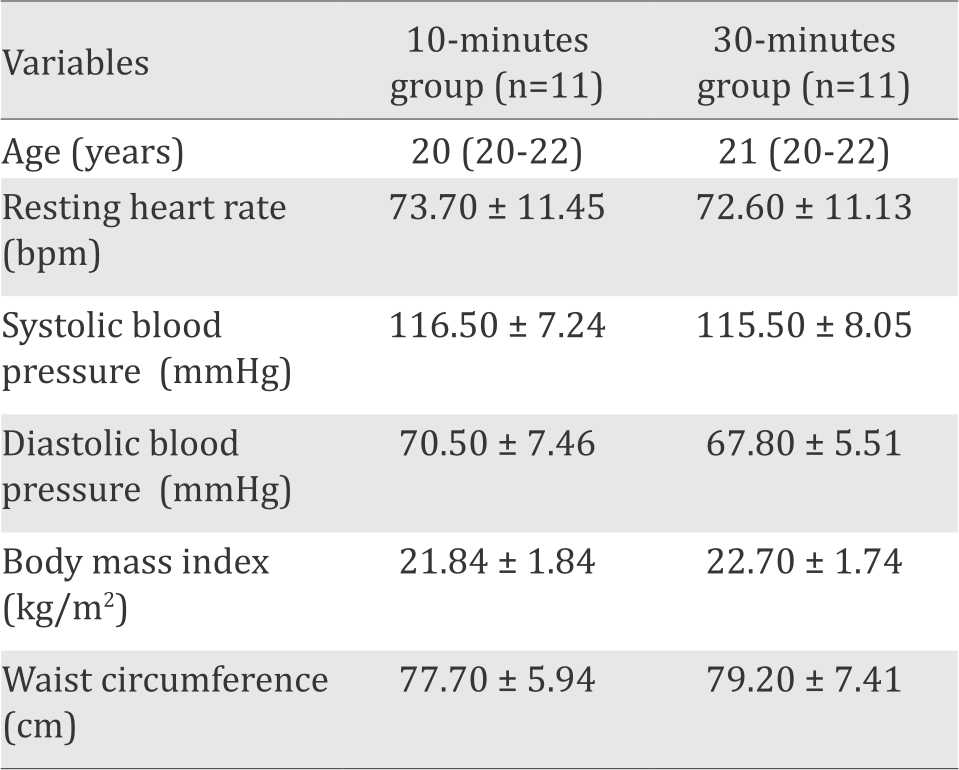
Data were presented as mean value ± standard deviations (SD), or as median (minimummaximum) for age data. Details of participant’s demographic variables are presented in Table 1. Unpaired t-test and Mann-Whitney test showed no significant difference between the two groups of physical exercise duration in terms of age, resting HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), and waist circumference. The results showed that the demographic data of both groups are homogenous.
Table 1. Demographic data of participants
Heart rate
Participants’ heart rate before and after static cycling treatment were presented in Figure 1. There was a significant difference between the mean heart rate before and after treatment in group of 10-minutes exercise, as well as in the group of 30-minutes exercise duration. However, there was no difference of post exercise heart rate between the two groups of exercise duration.
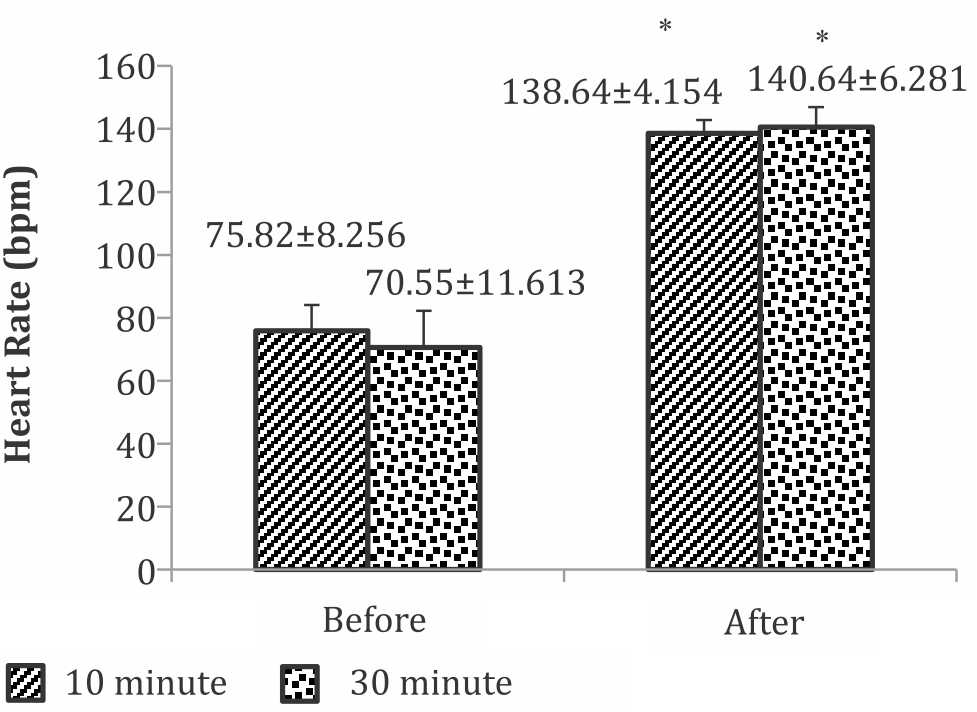
Figure 1. Heart rate before and after static cycling of 10 and 30-minute duration. *significant difference between the after treatment compared to the before treatment in each group of exercise duration (p<0.05)
Plasma GFAP and HSP27 levels
Plasma levels of GFAP and HSP27 before and after treatment for each group were presented in Figure 2. Figure 2a shows a slight but not significant increase of GFAP levels after static cycling of 10 minutes duration, whereas the HSP27 shows a significant decrease after exercise. Figure 2b depicts GFAP and HSP27 levels before and after treatment in the group of 30 minutes exercise duration. It showed a significant decrease in GFAP levels after exercise, whereas the decrease of HSP27 levels was not significantly different after treatment than before.
Figure 2c shows that post exercise plasma level of GFAP and HSP27 in the groups of 10 -minutes exercise was slightly higher than that of 30 minutes exercise duration. But the difference was not statistically significant.

Figure 2. Levels of plasma glial fibrillary acidic protein (GFAP) and heat shock protein 27 (HSP27). Panel A) GFAP and HSP27 levels before and after static cycling treatment of 10 minutes duration; B) GFAP and HSP27 levels before and after static cycling treatment of 30 minutes duration; C) GFAP and HSP27 levels after treatment of 10 or 30-minutes duration. *p<0.05
DISCUSSION
This study used treatment protocol based on ACSM aerobic physical exercise for healthy individuals aged 18–65 years, i.e a moderate intensity (64–74% HRmax) with duration of 10 minutes and 30 minutes.4 We choose static cycling for aerobic exercise because it does not require a high level of skill and prime level of fitness.4
Our results demonstrate that acute moderateintensity aerobic exercise of 10- and 30-minutes duration affect the plasma levels of GFAP and HSP27. The 10-minutes duration group showed a non-significant increased of GFAP levels but significant decreased of HSP27 levels. While the 30-minutes duration group showed significant decreased of GFAP levels and non-significant decreased of HSP27 levels.
Significant decrease of HSP27 plasma levels and non-significant increase of GFAP plasma levels on the 10-minutes duration group could be caused by several possibilities. The possibility is related with sub-optimal regulation of glucocorticoid hormones on HSP27 and GFAP. de Kloet et al12 revealed that peak levels of glucocorticoid hormones occurs after 15–30 minutes of physical or psychological stress (eg exercise). Similarly, a study conducted by Hill et al13 observed that blood cortisol increases after acute moderate-intensity aerobic physical exercise of 30-minutes duration. Glucocorticoid hormones regulate HSP27 and GFAP concentration.6,14 A study conducted by Barr et al6 showed that glucocorticoids increases HSP27 in brain tissue that gets stressors. Study conducted by Nichols et al14 proved that glucocorticoids regulate GFAP response by lowering its activity.
Sub-optimal level of glucocorticoid hormones result in increase of pro-inflammatory cytokines (IL1β) which will cause an increase in the expression of GFAP.14,15 Increase in proinflammatory cytokines upregulates Toll-Like Receptor (TLR)-4 expression on astrocytes membrane or TLR-2 expression on microglia membrane.16 This condition may contribute to binding of plasma circulating (extracellular HSP27) toward TLR-2 or TLR-4 as its recognize the receptor, thus resulting in the decrease of HSP27 plasma level.
The second possibility would be related with the increase in proinflammatory cytokines due to weak glucocorticoid action. And other possibility for the decrease in HSP27 is due to phosphorylation of HSP27.17,18 Phosphorylated HSP27 structures are oligomers that can not move out from the cell. Because more HSP27 are phosphorylated and can not move out from the cell, the plasma HSP27 level decreased.19 Reduced plasma HSP27 level causes weak interaction with GFAP, thus increasing GFAP plasma level. But the mechanism of oligomer formation still needs further studies.19 These results indicate that HSP27 used up early in interaction with reactive astrocytes as well as GFAP level. Astrocytes may not be able to effectively adapt to the 10 minutes of acute static cycling without the role of HSP27.
Significant decrease of GFAP plasma levels and non-significant decrease of HSP27 plasma levels on the 30-minutes duration group may be due to glucocorticoid hormones that have peaked. Increased glucocorticoid hormones cause an increase in HSP27 expression and a decrease in GFAP expression.6,14 An increase of glucocorticoid hormones also enhances the anti-inflammatory cytokines (IL-10),20 that would inhibit the expression of GFAP through inhibition of IL1β.15 Increased IL-10 also causes the extracellular HSP27 not being used as anti-inflammatory, and also causes inflammatory signals to decrease. These steps are assumed to decreased the expression of TLR so that there will be more HSP27 circulate in plasma.15,21,22 These results indicates that astrocytes was already able to adapt to the treatment of static cycling after 30 minutes.
GFAP and HSP27 plasma levels after treatment of 30-minutes duration were lower than that after treatment of 10-minutes duration, although not statistically significant. This contradictory result may be due to two possibilities. Firstly, the different duration of physical exercise might not have a different effect on the plasma levels of GFAP and HSP27. Secondly, the body (astrocytes) may have tried to maintain homeostasis resulting in a statistically non-significant difference. The absence of difference of post treatment groups of 10- and 30-minute durations, showed that the body responds differently to different stressor duration in order to obtain the same effect for the body. This is shown by the significant decrease of HSP27 level after aerobic exercise of 10-minutes duration, but after 30-minutes exercise, it was the GFAP that decreased significantly.
Lower levels of plasma GFAP and HSP27 after 30-minutes duration compared to that after 10-minutes, although non-significant, indicates that acute moderate-intensity aerobic exercise of 30-minutes duration is better to prevent the reactive astrocytes compared to the 10-minutes duration because the astrocytes seemed to be able to adapt after 30-minutes. However, this hypothesis needs further study. The ACSM recommended that an exercise duration of 10 minutes should be accumulated (3×10 minutes per day) and both of the duration of 10 minutes and 30 minutes should be performed in longterm (chronic).
This study was also unable to determine whether GFAP and HSP27 detected in plasma is originated from the brain, as there is no evidence on the correlation between GFAP and HSP27 in the brain and in plasma. Therefore, an animal study is needed to confirm the correlation between plasma and cerebral GFAP and HSP27 levels after one-session acute-moderate aerobic exercise.
In conclusion, acute moderate-intensity aerobic exercise of 10-minutes duration decreased HSP27 plasma levels, meanwhile 30-minutes duration of exercise decreased GFAP plasma levels. Acute moderate intensity aerobic exercise of 10- and 30-minutes duration has the same effect on the activity of astrocytes markers (GFAP and HSP27), indicating lowers astrocytes reactivity. But lower levels of those two parameters after 30-minutes duration of exercise compared to that of 10-minutes duration should be taken into consideration when choosing the exercise duration, because the 30-minutes duration reduces the reactivity of astrocytes more.
Conflicts of Interest
The authors affirm no conflicts of interest in this study
Acknowledgment
The authors thank to Margaretha, MD for financial, moral, and technical support on this study. The authors also thank Nelson, MD for the assistance in recruiting the participants.
REFERENCES
- World Health Organization, Geneva. Dementia: a public health priority. Available from: http://www.who.int/ mental_health/publications/dementia_report_2012/. [cited 4 July 2014].
- Bernardi C, Tramontina AC, Nardin P, Biasibetti R, Costa AP, Vizueti AF, et al. Treadmill exercise induces hippocampal astroglial alteration in rats. Neural Plast. 2013: 2013:1–10.
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–70.
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia: Lippincot Williams & Wilkins; 2010. 380p.
- Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93:421–43.
- Barr CS, Dokas LA. Glucocorticoids regulate the synthesis of HSP27 in rat brain slices. Brain Res. 1999;847:9–17.
- Periard JD, Ruell P, Caillaud C, Thompson MW. Plasma Hsp72 (HSPA1A) and Hsp27 (HSPB1) expression under heat stress: influence of exercise intensity. Cell Stress Chaperon. 2012;17:375–83.
- Perng MD, Cairns L, Van Den Ijssel P, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and aB-crystallin. J Cell Sci. 1999;112:2099–112.
- Mayer CA, Brunkhorst R, Niessner M, Pfeilschifter W, Steinmetz H, Foerch C. Blood levels of glial fibrillary acidic protein (GFAP) in patients with neurological diseases. Plos One. 2013;8(4):1–4.
- Vissers JLM, Mersch MEC, Rosmalen CF, Van Heumen MJ, Van Geel WJ, Lamers KJ et al. Rapid immunoassay for the determination of glial fibrillary acidic protein (GFAP) serum. Clin Chim Acta. 2006;366:336–40.
- Departemen Kesehatan Republik Indonesia Direktorat Jenderal Bina Kesehatan Masyarakat. Petunjuk teknis pengukuran kebugaran jasmani. Jakarta: Departemen Kesehatan RI; 2005. 62p.
- De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
- Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J. Endocrinol. 2008;31:587–91.
- Nichols NR, Agolley D, Zieba M, Bye N. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Res Rev. 2005;48:287–301.
- Woiciechowsky C, Schoning B, Stoltenburg- Didinger G, Stockhammer F, Volk HD. Brain IL-1β triggers astrogliosis through induction of IL-6: inhibition by propanolol and IL-10. Med Sci Monit. 2004;10(9):325–30.
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005; 175:4320–30.
- Ferns G, Shams S, Shafi S. Heat shock protein 27: its potential role in vascular disease. Int J Exp Pathol. 2006; 86:253–74.
- Satoh J, Kim Su. Cytokines and growth factors induce HSP27 phosphorylation in human astrocytes. J Neuropathol Exp Neurol. 1995;54(4):504–12.
- Garrido C, Paul C, Seigneuric R, Kampinga HH. The small heat shock proteins family: the long forgotten chaperones. Int J Biochem Cell B. 2012;44:1588–92.
- Tabardel Y, Duchateau J, Schmartz D, Marécaux G, Shahla M, Barvais L, et al. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men [abstract]. Surgery. 1996;119(1):76–80.
- Calderwood SK, Mambula SS, Gray PJ Jr, Theriault JR. Extracellular heat shock protein in cell signaling. FEBS Lett. 2007;581:3689–94.
- Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise. Exerc Sport Sci Rev. 2006;34(4):176–81.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id