
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Trehalose preincubation increases mesenchymal (CD271+) stem cells postcryopreservation viability
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i3.1273 Med J Indones. 2016;25:129–35
Received: August 23, 2015
Accepted: June 13, 2016
Author affiliation:
1 Faculty of Medicine, Universitas YARSI, Jakarta, Indonesia
2 Department of Anatomy, Physiology, and Pharmacology, Faculty of Veterinary Medicine, Bogor Agricultural University, Bogor, Indonesia
Corresponding author:
Indra Kusuma
E-mail: indralenycahaya@gmail.com
Background
Dimethyl sulfoxide (Me2SO) is a common cryoprotective agent widely used in cell preservation system. Me2SO is currently known to cause epigenetic changes which are critical in stem cells development and cellular differentiation. Therefore, it is imperative to develop cryopreservation techniques that protect cellular functions and avert Me2SO adverse effect. Trehalose was able to protect organism in extreme condition such as dehydration and cold. This study aimed to verify the protective effect of trehalose preincubation procedure in cryopreservation.
Methods
The study was conducted using experimental design. Thawed mesenchymal (CD271+) stem cells from YARSI biorepository were used for the experiment. Trehalose preincubation was performed for 1 hour, internalized trehalose was confirmed by FTIR-ATR measurement. Three groups consisted of (1) cryopreserved without trehalose preincubation, (2) cryopreserved with trehalose preincubation, and (3) did not undergo cryopreservation were evaluated after 24 hours in LN2 for viability in culture. The absorbance from each group was measured at 450 nm. The analysis performed using paired student t test.
Results
Viability of thawed mesenchymal (CD271+) stem cells that undergo trehalose preincubation prior cryopreservation was significantly higher (p<0.05) compared to group without trehalose preincubation. Higher viability observed between group with trehalose preincubation compared with controlled group suggests protection to trypsinization. Mesenchymal (CD271+) stem cells incubated for 1 hour in 100 mM trehalose supplemented medium results in 15% trehalose loading efficiency.
Conclusion
These findings confirm the protective effect of trehalose preincubation in cryopreservation. Future research should be directed to elucidate the trehalose internalization mechanism and eventually the protective mechanism of trehalose in mammalian cell cryopreservation.
Keywords
cryopreservation, mesenchymal (CD271+) stem cells, trehalose preincubation
Development of regenerative medicine using stem cells as an approach to replace damaged and worn out cells and tissues warrants a storage solution that protects stem cells unique ability to differentiate into many types of cells of the body. Current method of cell cryopreservation incorporated the use of dimethyl sulfoxide (Me2SO) as an intracellular cryomedium. This practice is currently under criticsm as described by Diaferia et al1 in their review. Me2SO is known to cause adverse effects and toxicity to patient, unexpected changes in cell fate, affects epigenetic control by acting on DNA methyltransferases, and loss of pluripotency in human embryonal stem cells.1 Despite the adverse effect of Me2SO, it is still commonly used as cell protectant thus we used Me2SO-based cryopreservation in this study.
Therefore, it is necessary to develop alternative procedure that can be adapted to cryopreservation workflow and avert the potential adverse effect of using Me2SO as an intracellular cryopreservant. Among other such as sucrose utilized by embryologist for ovum and sperm preservation, trehalose (378.33 g/mol) is a glucose disaccharides that has been recognized to be able to protect cells,2 organism3 and stabilize intracellular protein in extreme condition such as dehydration and cold.4 Cellular toxicity of trehalose was also compared in this study to give a picture of the extent of toxicity posed in trehalose preincubation to mesenchymal stem cells along with other disaccharides such as sucrose and known toxic chemicals such as Me2SO and hydrogen peroxide which is a potent oxydator and caused damage to organic compound. Preincubation was known to lead to trehalose internalization into the cytoplasm. Several methods of trehalose internalization requires cell poration using adenosine triphosphate (ATP) and the use of liposomes is being investigated.5–7
Mesenchymal stem cell is a heterogeneous population recently discovered to contain not only multipotent stem cell but also non-tumorigenic pluripotent stem cell subpopulation known as the multi-lineage differentiating stress enduring (MUSE) cells.8,9 The ability to preserve functional properties of such unique subpopulation will greatly contribute to the development of regenerative medicine.
Trehalosa as a cryoprotectant has been evaluated in several types of cells such as mesenchymal stem cells-derived from bone marrow, erythrocytes, platelets, and umbilical cord blood stem cells.10–12 Suggested method of trehalose internalization was by fluid phase endocytosis after 1–24 hours of trehalose pre-treatment via clathrin-mediated endocytosis.12 However, this was suggested as cell-type specific because the endocytosis mechanism involved.13 This study aimed to verify the protective effect of trehalose-preincubation in Me2SO-based cryopreservation of mesenchymal (CD271+) stem cells derived from peripheral blood mononuclear cells which were a potential source of MUSE cells.
METHODS
Research design
The present study conducted using experimental design from December 2014 to March 2016 at YARSI University Cell Culture Facility, Jakarta, Indonesia. Ethical approval received from Komisi Etik Lembaga Penelitian Universitas YARSI (No. 016/KEP-UY/BIA/II/2016).
Cell culture
Mesenchymal stem cells were derived from plasticadherent mononuclear cells of peripheral blood (PBMC) obtained from Laboratorium Terpadu Universitas YARSI Biorepository. Purified using CD271 magnetic sorting magnetic-activated cell sorting (MACS); Miltenyi, expanded and cultured in Dulbeco’s modified eagle’s medium (DMEM) low glucose (Gibco) supplemented with 10% fetal bovine serum (FBS) heat inactivated (Gibco). 1% penicillin-streptomycin and fungizone (Gibco) added to prevent bacterial and fungal contamination. Mesenchymal stem cells used for experiments were from passage four to six in exponential phase. All experiment described below were performed in triplicate.
Toxicity of trehalose, sucrose, Me2SO and hydrogen peroxide were compared using CD271+ mesenchymal stem cells. The study was conducted in a 96 microwell plate with as much as 20,000 viable cell cultured. Incubation of the investigated chemicals at 0 mM, 12.5 mM, 25 mM, 50 mM, and 100 mM of trehalose, 0 mM, 18.75 mM, 37.5 mM, 75 mM, and 150 mM of sucrose, 0%, 0.06%, 0.12%, 0.25%, 0.5%, and 1% of Me2SO and 0 μM, 31.25 μM, 62.5 μM, 125 μM, 250 μM, 500 μM hydrogen peroxide for one hour and continued with 30 minutes water-soluble tetrazolium-1 (WST-1) proliferation assay (Roche) incubation to assess cell viability. Measurement conducted using microplate reader at 450 nm. Long-term toxicity was assessed in seven-days-culture, viability was measured at three different time point (day in vitro/DIV two, four, and seven) also using WST-1 proliferation assays in 96 microwell plate. Osmolarity measurement of medium supplemented by trehalose and sucrose were conducted at Faculty of Veterinary Medicine, Bogor Agricultural University, Embryology Laboratory.
Trehalose measurement by FTIR-ATR
Mesenchymal (CD271+) stem cells cultured at 80% confluency using tissue flask 25 sqcm were incubated with 100 mM trehalose mixed in culture medium for one hour. Cells were harvested by trypsinization using trypsin / ethylenediaminetetraacetic acid (EDTA) 0.05% for five minutes and washed by centrifugation twice. Resulting pellet were counted using tryphan blue exclusion method and adjusted for 1,000,000 viable cell resuspended in 50 μl phosphate buffer saline (PBS), Gibco for FTIR measurement. Intracellular trehalose detection performed using fourier transform infrared spectroscopy (FTIR) – attenuated total reflectance (ATR).
Cell pellet were dried using deoxyribonucleic acid (DNA) concentrator (MiVac) at 37°C for 45 minutes. Infrared spectra were generated by putting dried cell pellet on top ATR germanium crystal. 32 scan performed by FTIR at 1,100–900 wavenumber/cm, peak at 995–991 wavenumber/ cm as reported by Sakurai et al14 was considered as trehalose. Trehalose concentration was determined using simple Beer’s law principle by generating trehalosa standard curve in FTIR-ATR (Nicolet-Bruker). Omnic software was used to measure peak height and area under peak curve to generate a standard curve of known trehalose concentration with a linear fitting.
Cryopreservation
Trehalose-incubated mesenchymal (CD271+) stem cells described previously were cryopreserved using standard slow-freeze technique and cryoprotectant (70% culture medium, 20% Me2SO, and 10% fetal bovine serum). Three groups were established consist of (1) cryopreserved without trehalose preincubation-slow freeze 721 (SF721) (group 1), (2) cryopreserved with trehalosepreincubation- SF721-Tre (group 2), and (3) did not undergo cryopreservation (control). Cells were stored in -80°C overnight and transferred to liquid nitrogen (LN2) the next day. After 24 hours in LN2 cells were thawed and cultured in 96 microwell plate at 10,000 viable cells per well for functional evaluation using WST-1 proliferation assay. Absorbance of attached and viable cell after 60 minutes plating were measured at 450 nm.
Data analysis
Blank absorbances were subtracted from the original data. Analysis was performed using Excell (version 2013; Microsoft). Statistical significance between group 1 and control group and between group 2 and control group were both determined using paired student t test. Phosphate buffer saline spectra were subtracted from all spectra obtained to construct the trehalose standard curve.
RESULTS
Toxicity studies
Toxicity profile of Me2SO, trehalose, and sucrose hydrogen peroxides were illustrated in Figure 1. These data suggest that 100 mM of trehalose was at comparable toxicity to 1% Me2SO which was currently accepted as a safe level for cell exposure. A minimum of 150 mM of sucrose was also comparable but the osmolality limited it used. The level of toxicity posed by these dissacharides is comparable with a very low level of hydrogen peroxide concentration.
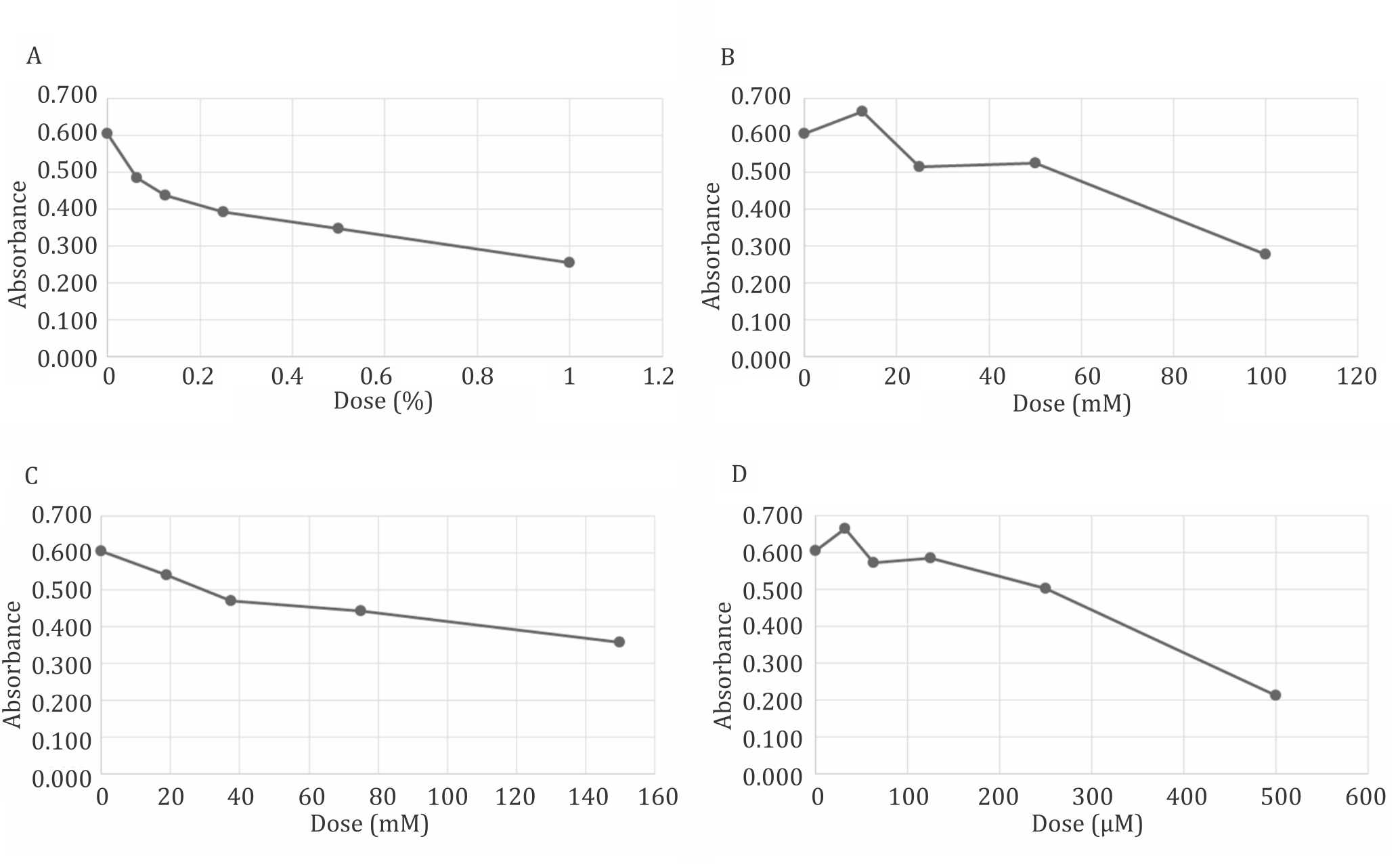
Figure 1. Toxicity profile of Me2SO (A), trehalose (B), sucrose (C), and hydrogen peroxide (D) to mesenchymal (CD271+) stem cells. Toxicity of 100 mM of trehalose is relative comparable to 1% Me2SO, 160 mM sucrose and 500 μM of hydrogen peroxide. No significant difference (p>0.05) between trehalose (100 mM) and Me2SO (1%)
Further evaluation of the disaccharides were conducted using 50 mM, 25 mM, and 12.5 mM of trehalose and sucrose. Compared to control (0mM) group, within each time points both disaccharides reduced cells viability in a dosedependent manner. However, observation of each dose group from three different time points during seven days of culture showed that the cells continued to have significant proliferation capacity despite the presence of disaccharides in the medium (Figure 2). Disaccharides toxicity was considered related to cellular hydration status. Figure 3 shows the changes in culture medium osmolality after supplementation with trehalose and sucrose. Increase in medium osmolality caused a relative hyperosmolarity in extracellular environment and triggered water efflux from intracellular compartment that caused dehydration and cell death.
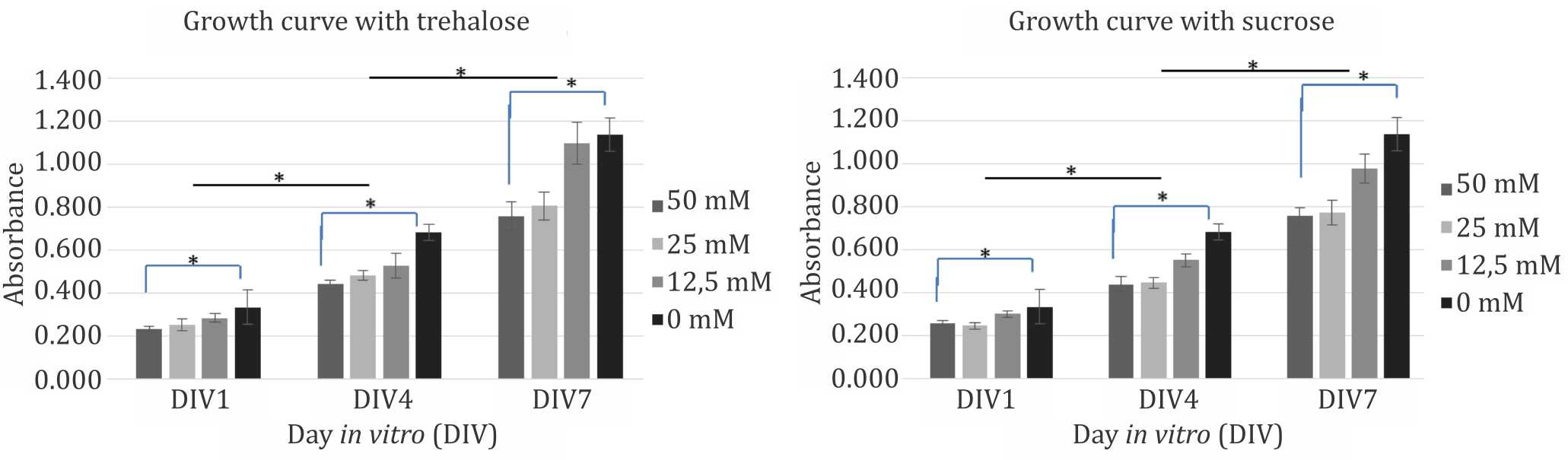
Figure 2. Growth curve of mesenchymal (CD271+) stem cells with trehalose (left) and sucrose (right) as suplementation in culture medium. * p<0.05
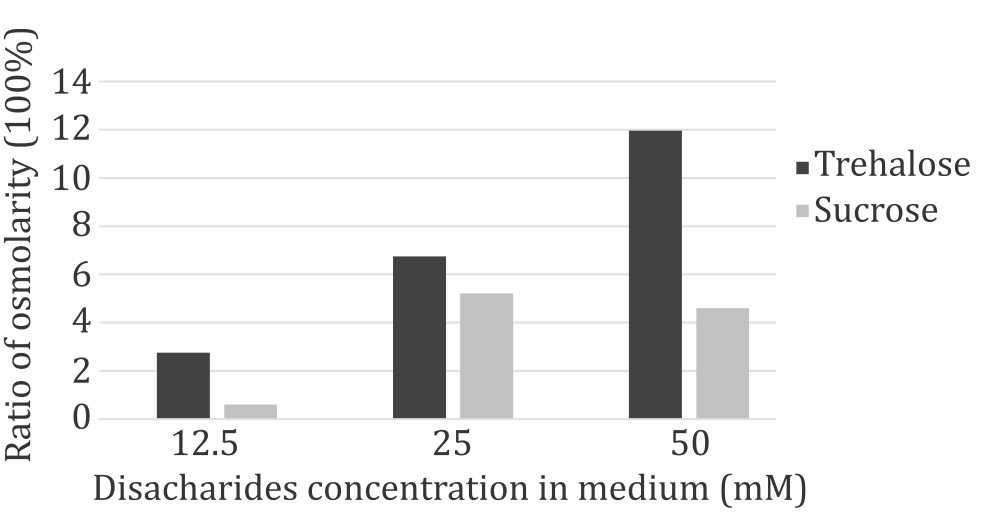
Figure 3. Changes in medium osmolarity after disacharides suplementation
Intracelullar trehalose measurement
Internalization of trehalose into cytoplasm was confirmed by FTIR measurement. Figure 4 shows a spectrum recorded from washed and dried cell pellet with characteristic peak at the fingerprint region (below 1,500/cm) for trehalose at 991–992 /cm. Absorbance level of trehalose derived from infrared spectra were used to construct trehalose standard curve for intracellular trehalose quantitation. (Figure 5) the standard curve allowed quantitation of trehalose within the cytoplasm which gives a resultof 15% loading efficiency. 15.07 mM of trehalose were estimated within the cytoplasm of approximately 1,000,000 viable cells that were measured by FTIR-ATR.
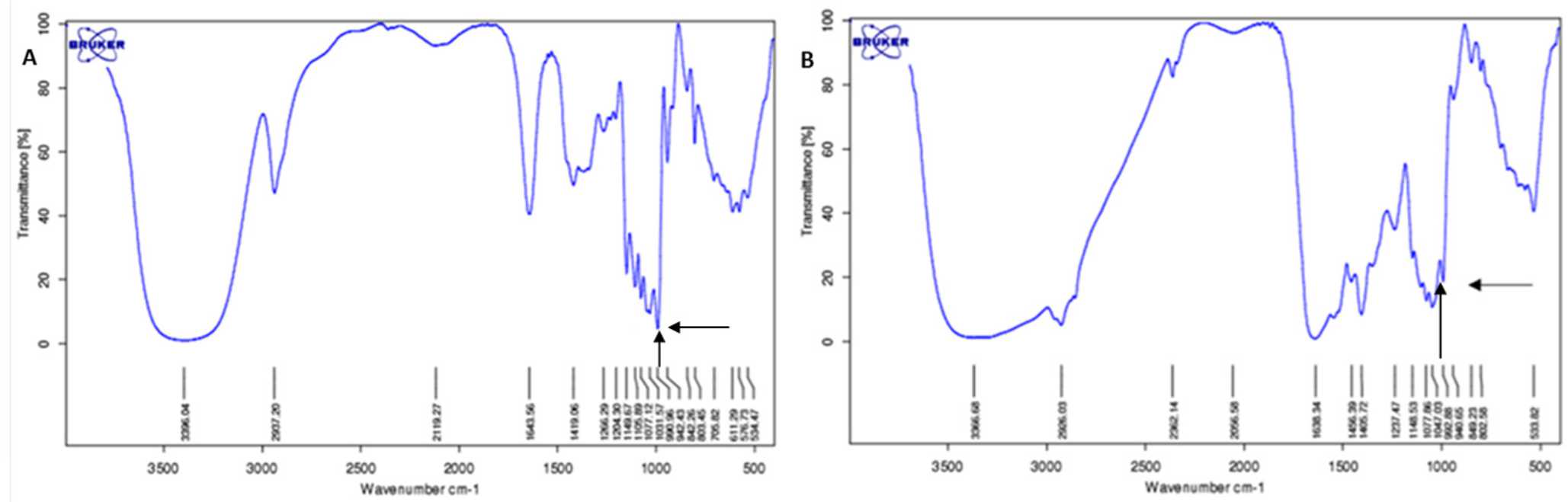
Figure 4. A) Spectrum recorded from 250 mM trehalose solution shows a characterisitic peak of trehalose at 990.96 /cm wavenumber (arrow); B) Spectrum of dried lysate cell derived from 100 mM trehalose preincubated cell culture shows trehalose peak at 992.65 /cm wavenumber (arrow). The difference in wavenumber attributed to water content from the trehalose solution
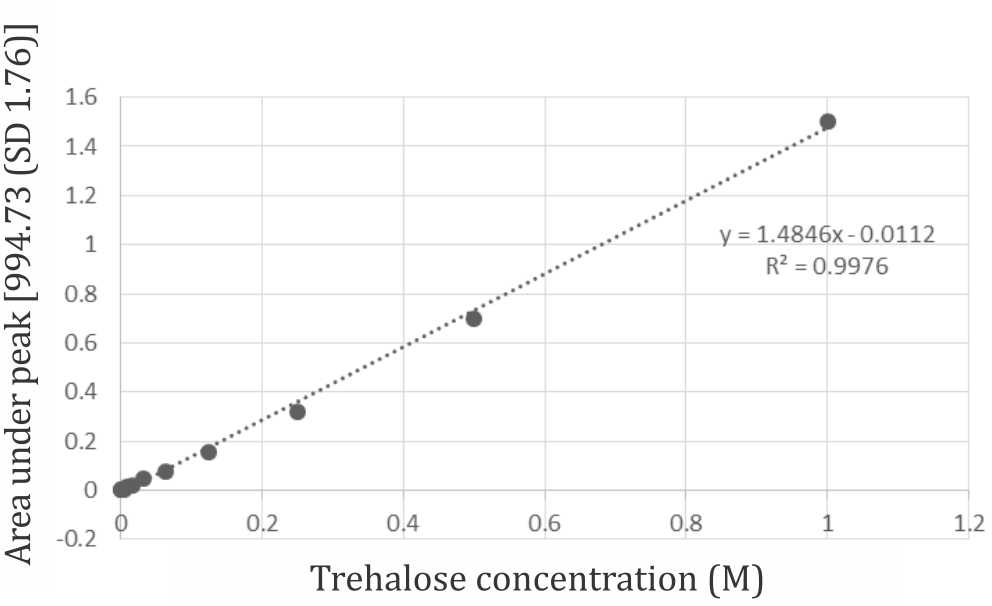
Figure 5. Trehalose standard curve with linear fitting
Cryopreservation study
Trehalose-incubated cells showed a greater (p<0.05) viability and attachment after 24 hours in liquid nitrogen compared with nontrehalose- incubated cells and control group which was not cryopreserved. These findings confirmed that intracellular trehalose were able to protect mesenchymal (CD271+) stem cells in cryopreservation. Figure 6 shows significant viability retained by trehalose-incubated cells compared to non-cryopreserved cells (control group). These data suggested a certain protection against stress evoked by trypsinization process.
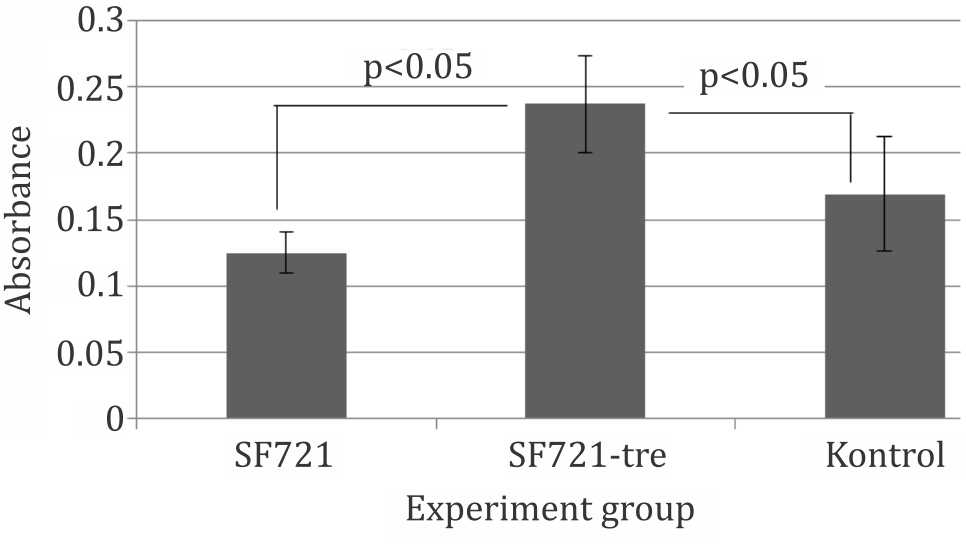
Figure 6. Viability of attached cells in culture after thawing. SF721: Cryopreserved without trehalose preincubation; SF721-Tre: Cryopreserved with trehalose preincubation; SF721: slow-freeze with 70% culture mediun, 20% FBS and 10% Me2SO
DISCUSSION
Our data showed that extracellular trehalose could cause a toxic effect on cells by inducing osmolality imbalance. Lynch et al15 reported approximately 60% of cells death recorded after cells loaded with over 200 mM of internal trehalose concentration were resuspended in phosphate buffer saline of physiologically osmolality (300 mOsm). Our toxicity data showed a low dose of extracellular trehalose (<50 mM) caused reduction in viability at day seven of culture (Figure 2). However, an increase in viability was clearly observed compared to previous time point in the same dose group. This suggests that trehalose toxicity did not affect all of the cells population and the mesenchymal stem cells population still retained the proliferative capacity.
Zhou et al2 used 50 mM trehalose incubated for four hours results in 13 mM intracellular trehalose concentration which quite similar with our data (15.07 mM). Zhang et al16 also have a similar result of 14.57 mM while Oliver et al12 reported at 19 mM. The use of ATP as a poration agent greatly increased the loading kinetics such as intracellular concentration of 50 mM reached after 90 minutes incubation.6 These data were derived from different cell and different method of quantitation such as anthrone reaction and HPLC measurement. As far as we know our study is the first that used FTIR-ATR for intracellular trehalose quantification.
Our study suggests that trehalose-preincubation which results a 15 mM of intracellular trehalose concentration alongside the standard slowfreezing, Me2SO-based cryomedium were sufficient to protect mesenchymal (CD271+) stem cells from cryoinjury. Although to achieve this we used 100 mM of trehalose with one hour preincubation protocol which caused significant reduction in cell viability. This condition warrants a further investigation in trehalose internalization mechanism. Furthermore, our data suggest that intracellular trehalose was benefit in preventing trypsinization-induced cell injury. Trypsin, although widely used in passaging procedure, is in fact a protease that can cause stress and cell death. Trehalose was known to help retain cellular integrity and prevent protein denaturation.4 Therefore, intracellular trehalose may prevent cell injury during trypsinization resulting in a higher viability compared to the control group.
Different trehalose loading kinetics reported by comparing different study that has been conducted on platelets, erythrocytes, and stem cells using trehalose incubation method, suggest there is a cell-type specific of internalization method. Although Oliver et al12 reveals bonemarrow derived mesenchymal stem cells used clathrin-mediated endocytosis for trehalose internalization, this not necessarily the same for other cell type. Elucidating the mechanism of trehalose internalization of different cell type can shed a new understanding of cell membrane regulation.
In conclusion, this study concluded that trehalose-preincubation procedure was able to protect mesenchymal (CD271+) stem cells from cryoinjury results from slow-freeze cryopreservation procedure. Further study should be directed to elucidate factors that regulate trehalose internalization kinetics in different cell types. Research could also be directed to verify trehalose cryoprotection in different type of cryopreservation such as vitrification.
Conflicts of Interest
Funding was provided by Lembaga Pengelola Dana Pendidikan (LPDP) scholarship scheme.
Acknowledgment
Funding was provided by LPDP scholarship scheme. We thank Anna Roswiem and Triayu Septiani for assistance in operating FTIR-ATR measurement, and Intan Razari for her assistance in using MiVac DNA concentrator.
REFERENCES
- Diaferia GR, Dessi SS, DeBlasio P, Biunno I. Is stem cell chromosomes stability affected by cryopreservation conditions? Cytotechnology. 2008;58(1):11–6.
- Zhou XL, Zhu H, Zhang SZ, Zhu FM, Chen GM, Yan LX. Freeze-drying of human platelets: influence of intracellular trehalose and extracellular protectants. Cryo Letters. 2006;27(1):43–50.
- Streeter JG. Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J Appl Microbiol. 2003;95(3):484–91.
- Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36.
- Emanuele E, Bertona M, Sanchis-Gomar F, Pareja- Galeano H, Lucia A. Protective effect of trehalose-loaded liposomes against UVB-induced photodamage in human keratinocytes. Biomed Rep. 2014;2(5):755–9.
- Elliott GD, Liu XH, Cusick JL, Menze M, Vincent J, Witt T, et al. Trehalose uptake through P2X7 purinergic channels provides dehydration protection. Cryobiology. 2006;52(1):114–27.
- Lynch AL, Chen R, Slater NK. pH-responsive polymers for trehalose loading and desiccation protection of human red blood cells. Biomaterials. 2011;32(19):4443–9.
- Tsuchiyama K, Wakao S, Kuroda Y, Ogura F, Nojima M, Sawaya N, et al. Functional melanocytes are readily reprogrammable from multilineagedifferentiating stress-enduring (muse) cells, distinct stem cells in human fibroblasts. J Invest Dermatol. 2013;133(10):2425–35.
- Simerman AA, Perone MJ, Gimeno ML, Dumesic DA, Chazenbalk GD. A mystery unraveled: nontumorigenic pluripotent stem cells in human adult tissues. Expert Opin Biol Ther. 2014;14(7):917–29.
- Lynch AL, Slater NK. Influence of intracellular trehalose concentration and pre-freeze cell volume on the cryosurvival of rapidly frozen human erythrocytes. Cryobiology. 2011;63(1):26–31.
- Rodrigues J, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56(2):144–51.
- Oliver AE, Jamil K, Crowe JH, Tablin F. Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv Tech. 2004;2(1):35–49.
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–33.
- Sakurai M, Furuki T, Akao K, Tanaka D, Nakahara Y, Kikawada T, et al. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc Nat Acad Sci. 2008;105(13):5093–8.
- Lynch AL, Slater NK. Mediated trehalose un-loading for reduced erythrocyte osmotic fragility and phosphatidylserine translocation. Cryo Letters. 2011;32(5):415–24.
- Zhang S, Qian H, Wang Z, Fan J, Zhou Q, Chen G, et al. Preliminary study on the freeze-drying of human bone marrow-derived mesenchymal stem cells. J Zheijang Univ. 2010;11 (11):889–94.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id