
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Pathological Q wave as an indicator of left ventricular ejection fraction in acute myocardial infarction
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i2.1274 Med J Indones. 2016;25:98–103
Received: September 01, 2015
Accepted: June 07, 2016
Author affiliation:
1 Faculty of Medicine, Universitas Mulawarman, Samarinda, Indonesia
2 Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Mulawarman, Samarinda, Indonesia
3 Laboratory of Public Health, Faculty of Medicine, Universitas Mulawaman, Samarinda, Indonesia
Corresponding author:
Muhammad S. Tiyantara
E-mail: suryatiyantara@gmail.com
Background
Q-wave myocardial infarction (QMI) has higher mortality and lower myocardial viability than non-Qwave myocardial infarction (NQMI), suggesting the existence of pathological Q waves reflects the worse ventricular function. The aim of the study is to determine difference in left ventricular ejection fraction (LVEF) between QMI and NQMI.
Methods
The study design was cross-sectional analysis conducted in patients with AMI that were hospitalized and undergone echocardiography in Abdul Wahab Sjahranie County General Hospital Samarinda during February 2014 to March 2015. Standard 12-lead electrocardiograms (ECG) were recorded at presentation, 1 day and 2 days after the onset of AMI as well as using the classical criteria for pathological Q wave. LVEF assessment was performed using echocardiography after the second day since the onset of AMI. Independent-T test was used to determine difference in LVEF using PSPPIRE 0.8.4.
Results
There were 34 subjects comprising 16 QMI patients and 18 NQMI patients. QMI had a lower LVEF (42±13%) compared to NQMI (60±11%, p<0.001). The presence of pathological Q waves was associated with LVEF ≤40% (p=0.002).
Conclusion
QMI had a lower LVEF than NQMI, provides information about the role of pathological Q wave as an indicator of LVEF.
Keywords
LVEF, non-Q-wave myocardial infarction, pathological Q wave, Q-wave myocardial infarction
Acute myocardial infarction (AMI) can be classified into Q-wave myocardial infarction (QMI) and non-Q-wave myocardial infarction (NQMI) based on the presence of pathological Q waves.1 Most QMI is a final diagnosis of STelevation myocardial infarction (STEMI) and a small portion is a final diagnosis of non-STelevation myocardial infarction (NSTEMI).2 Myocardial viability is lower in QMI.3,4 Mortality and the incidence of congestive heart failure and cardiogenic shock are higher in QMI.5-7
Pathological Q wave in the AMI is an abnormal negative deflection in electrocardiogram (ECG) which indicates a significant cardiac electrical abnormality.1,8,9 The conditions that responsible in the formation of pathological Q wave are myocardial necrosis, hibernation, and stunning, those are known as the causes of ventricular contractile dysfunction.3,8-11
Left ventricular ejection fraction (LVEF) is an indicator of ventricular function that can be used to assess ventricular systolic function in AMI patients. Left ventricular systolic function can be measured non-invasively using echocardiography as LVEF.
Because there were evidences that pathological Q wave reflecting the abnormal conditions of myocardium, we had hypothesized that pathological Q wave had a potential as an indicator of LVEF. The aim of this study was to determine difference in LVEF between QMI and NQMI.
METHODS
This study was cross-sectional analysis. The subjects were patients with AMI for the first time who were treated and undergone echocardiography in period of February 2014 to March 2015 in the Abdul Wahab Sjahranie Samarinda County General Hospital as the referral center in East Kalimantan. The thrombolytic therapy, anticoagulant, antiplatelet and other medical therapy were used as standard therapy as indicated. All data were obtained from medical record archives. AMI was defined as the presence of manifestation of acute coronary syndrome, cardiac biomarker rise above the upper limit of normal values based on local laboratory, and evidence of ECG that support the diagnosis. Subjects with QRS confounders (such as left bundle branch block), and/or poor quality ECG were excluded.
The diagnosis of QMI was based on the appearance of pathological Q waves in AMI in one or more ECG measurement in the specific time with the typical evolution pattern of infarction. The specific time defined as the first three measurements of the ECG that include at presentation, one day, and two days after the onset of AMI. Standard 12-lead ECGs were recorded at specific time in order to bring enough time to evaluate the appearance of pathological Q wave, thus, enabling to determining the final diagnosis of the type of infarction and to evaluate the typical evolution pattern of infarction. The pathological Q wave was defined by using classical criteria for pathological Q wave that defined as Q-wave with a duration ≥40 ms and/or a depth ≥25% of the R-wave in the same lead or the presence of a Q wave equivalent, the Q wave must be contained in ≥2 leads to the same lead group. When the pathological Q waves were present in the one or more ECG measurement in the first three ECG measurements (at presentation, one day, and two days after the onset of AMI), then, the diagnosis was QMI, if the waves were not present, the diagnosis was NQMI (Figure 1).
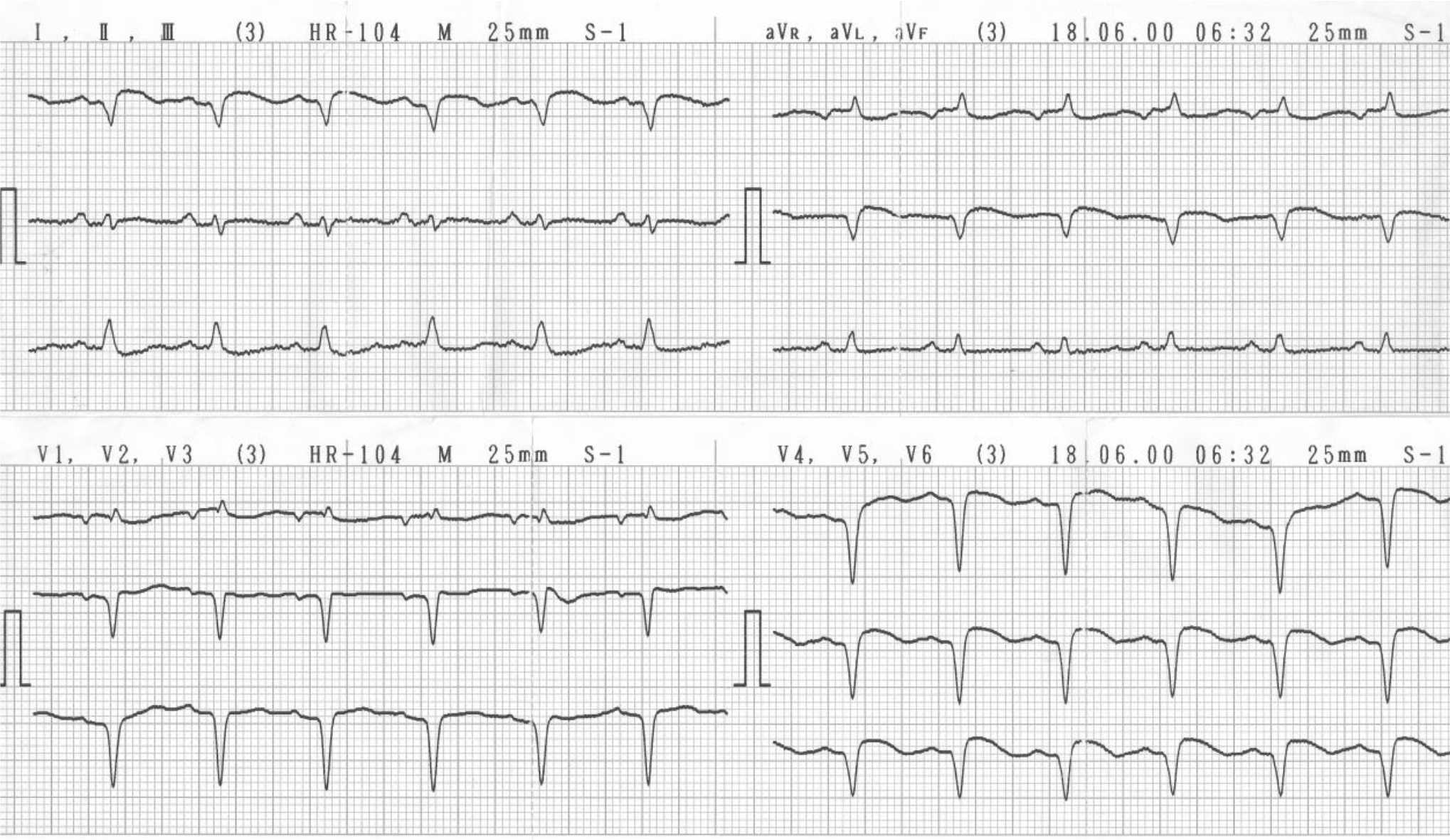
Figure 1. Subject with left ventricular ejection fraction (LVEF) 22%. Pathological Q waves in I, aVL, V2, V3, V4 , V5, and V6
The LVEF examination was performed using echocardiography. The assessment was carried out by operators who did not know the status of participation of the subjects in the study. LVEF assessment technique using the M-mode (1-D) echocardiography or Simpson’s method. Philips© echocardiography machines were used in this study. The LVEF examination was performed after the second day since the subjects were hospitalized.
Continuous variables were expressed as mean and standard deviations for data that not skewed or median (25th percentile, 75th percentile) for skewed data. Categorical variables were expressed as frequency with percentage. Independent T test was used to compare the characteristics expressed as continuous variables. The chi square test was used to compare noncontinuous variables. Statistical significance is obtained when p<0.05. All analyses were done using PSPPIRE 0.8.4.
The study protocol was approved by the Research Ethics Committee on Faculty of Medicine Universitas Mulawarman (No. 74/KEPK-FK/ IV/2015).
RESULTS
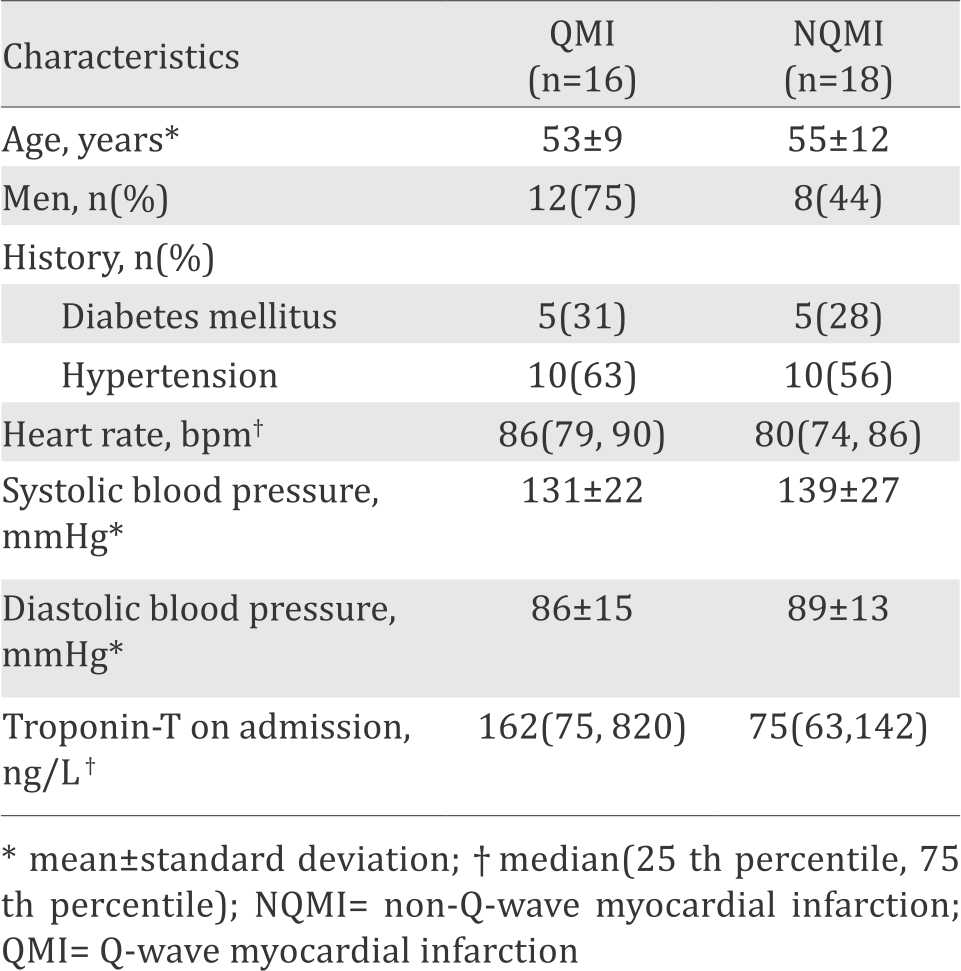
During the study, we recruited 34 subjects consisting of 16 QMI patients and 18 NQMI patients. The subject characteristics at hospital admission are shown in Table 1. The youngest age was 31 years, while the oldest was 74 years with mean age of the subjects was 54±11 years (Table 1).
Table 1. Subject characteristics
Approximately 30% of the LVEF assessments were done using Simpson’s method. The mean LVEF of all subjects was 52±15%, the lowest value (22%) was contained in the QMI subject (Figure 1) while the highest LVEF (81%) was contained in the NQMI subject. Analysis of LVEF difference between QMI and NQMI was performed using Independent T test. QMI had a lower LVEF than NQMI (42±13% and 60±11%, p<0.001). A total of 56% of the QMI subjects had LVEF ≤40%, while 6% of the NQMI subjects had the value (p=0.002).
DISCUSSION
Acute myocardial infarction causes changes in myocardial performance. The decreased blood flow causes electrical and mechanical disturbances in the myocardium. Significant electrical disturbances can lead to appearance of pathological Q waves on ECG.1
Pathological Q wave is associated with a state of myocardial hibernation, stunning, and necrosis.3,8-11 Severe blockage of myocardial blood flow in a long time tends to cause myocardial necrosis while the lighter flow allows the myocardium last longer through the mechanism of myocardial hibernation though both can cause myocardial contractile dysfunction.12,13 The contractile dysfunction can also occur despite reperfusion has been done with the flow of blood that has been normal or nearly normal in the condition of myocardial stunning.14 The extent of myocardial damage is correlated to the pathological Q wave amplitude and the decreased ventricular contractile work.15,16
Our study confirmed the role of pathological Q wave as an indicator of LVEF. As the pathological Q waves appeared in ECG at the specific time, then, the lower LVEF was suggested. This study also found the association between the appearance of the waves and LVEF ≤40%. These findings confirm the theory of the conditions that responsible in the formation of the pathological Q wave that potentially lowering the ventricular function. The lower LVEF in QMI also confirms the prognosis role of the pathological Q wave, as the worse prognosis is prominent in subject with reduced ventricular function in AMI.
Another study by Delewi et al17 showed similar results, the subjects were STEMI patients undergoing primary percutaneous coronary intervention (PPCI) where QMI had a lower LVEF than NQMI (37±8% and 45±8%, p=0.001). Although they used different subjects and used cardiac magnetic resonance (CMR) to calculate the LVEF, their results also showed the similar meaning of the appearance of pathological Q waves, the similar pathophysiological mechanism may be responsible in this situation.
A study by Tao et al15 found a negative correlation between LVEF and pathological Q wave amplitude in experimental animals. This finding suggests that the pathological Q waves are not only the indicator of the lower LVEF but also tell us the severity of the reduced LVEF, but a study in human is still needed. Our study did not evaluate the meaning of the amplitude as we focus in the appearance of the wave.
In contrast, study by Yang et al18 showed there was no difference in LVEF between QMI and NQMI (25±11% and 28±10%, p>0.05), their subjects were patients with history of AMI (more than one month after AMI) with ventricular dysfunction.18 The difference of their result with our finding may be resulted from many factors, such as the difference of subject selection criteria (we used subjects with the first AMI event), the time of LVEF assessment (time-dependent ventricular remodelling), pathological Q waves regression events, the recovery of stunned myocardium, ischemic preconditioning events, and the timing and types of the treatment.
There was difference in LVEF between NSTEMI and non-Q-STEMI (55.5±9.5% and 46.6±7.3%, p<0.001), and between NSTEMI and Q-STEMI (55.5±9.5% and 43.1±7.8%, p<0.001), but no difference was found between non-Q-STEMI and Q-STEMI (46.6±7.3% and 43.1±7.8%, p>0.05) on study by Plein et al.19
There are several theoretical explanations to our results. The classical criteria that we used in our study are associated with size of infarction.17 Study by Pride et al16 showed LVEF had negative correlation with the size of infarction.16 Subjects without pathological Q wave have the lowest size of infarction and subjects with pathological Q wave in line with the increase in Q wave amplitude have the increasing infarct size,15,20 NQMI has myocardial necrosis amount sufficient to increase the value of biomarkers of myocardial damage but not enough to produce abnormal deflection in ECG namely pathological Q waves.1
Myocardial necrosis has a role as the condition that makes QMI had a lower LVEF. Myocardial necrosis has long been known as the condition that responsible for the formation of pathological Q waves. Electrophysiological mechanisms of formation of pathological Q waves are explained through the theory of electrical window using zones of necrosis.9 Subjects with pathological Q waves have a lower myocardial viability than subjects without pathological Q waves on an assessment using the dobutamine stress echocardiography (DSE).3 Study conducted by Ananthasubramaniam et al4 showed subjects with pathological Q wave had more myocardial scars.
Hibernation and myocardial stunning are conditions that also have a role in the formation of pathological Q waves and the decrease in LVEF. A Study that conducted by Sztajzel and Urban10 stated that the pathological Q waves did not only indicate myocardial necrosis but also stunning and hibernation, this was evidenced by the presence of pathological Q waves reversibility. Study by Delewi et al17 showed that regression of pathological Q waves was an indicator of the increase in LVEF that also showed the reversibility of pathological Q waves as an indicator of reversible myocardial dysfunction.17 Study conducted by Voon et al11 using Tl-201 myocardial perfusion single photon emission computed tomography (SPECT) showed that regression of pathological Q wave indicating an improvement of hibernation and/or stunning.11
This study had several limitations. The number of subjects was small. The techniques used for LVEF assessment for AMI patients in Abdul Wahab Sjahranie hospital were varied, and M-mode (1-D) echocardiography technique was dominantly used in this study. M-mode (1-D) echocardiography is not accurate than other echocardiography technique (2-D Simpson’s method and 3-D echocardiography) especially in conditions where ventricular geometry is not uniform.21,22 Myocardial viability was not assessed in this study so the underlying conditions (hibernation, stunning, and necrosis) that responsible in the decrease of LVEF cannot be determined clearly. However, our result can be more generalized for the AMI patient that undergone non-PPCI management such as thrombolytic therapy, anticoagulant, antiplatelet and/or other medications that still be used in many settings.
In conclusion, the existence of pathological Q waves reflected the worse ventricular function. It provides information about the role of pathological Q wave as an indicator of ventricular function especially LVEF in AMI. The appearance of the waves not only can predict the lower LVEF but also should be the sign of indication of more aggressive treatment and evaluation as the poor ventricular function has a worse prognosis in the short-term and long-term period.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
None.
REFERENCES
- Velasco M, Rojas ER. Non-Q-wave myocardial infarction: comprehensive analysis of electrocardiogram and pathological correlation. Síndrome Cardiometabólico. 2011; 1(1):4–10.
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/ non–ST-elevation myocardial infarction. Circulation. 2013;127:663–828.
- Jeon HK, Shah GA, Diwan A, Cwajg JM, Park TH, McCulloch ML, et al. Lack of pathologic Q waves: a specific marker of viability in myocardial hibernation. Clin Cardiol. 2008;31(8):372–7.
- Ananthasubramaniam K, Chow BJ, Ruddy TD, deKemp R, Davies RA, DaSilva J, et al. Does electrocardiographic Q wave burden predict the extent of scarring or hibernating myocardium as quantified by positron emission tomography? Can J Cardiol. 2005;21(1):51–6.
- Halkin A, Fourey D, Roth A, Boyko V, Behar S. Incidence and prognosis of non-Q-wave vs. Q-wave myocardial infarction following catheter-based reperfusion therapy. QJM. 2009;102(6):401–6.
- Armstrong PW, Fu Y, Westerhout CM, Hudson MP, Mahaffey KW, White HD, et al. Baseline Q-wave surpasses time from symptom onset as a prognostic marker in STsegment elevation myocardial infarction patient treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2009;53(17):1503–9.
- Siha H, Das D, Fu Y, Zheng Y, Westerhout CM, Storey RF, et al. Baseline Q waves as a prognostic modulator in patients with ST-segment elevation: insight from the PLATO trial. CMAJ. 2012;184(10):1135–42.
- Bonow, Mann, Zipes, Libby. Braunwald’s heart disease: a textbook of cardiovascular medicine. 10th ed. Philadelphia: Elsevier; 2015.
- Camm AJ, Lüscher TF, Serruys PW. The ESC textbook of cardiovascular medicine. 2nd ed. Oxford: Blackwell Publishing; 2006.
- Sztajzel J, Urban P. Early and late Q wave regression in the setting of acute myocardial infarction. Heart. 2000;83(6):708–10.
- Voon WC, Chen YW, Hsu CC, Lai WT, Sheu SH. Q-wave regression after acute myocardial infarction assessed by Tl-201 myocardial perfusion SPECT. J Nucl Cardiol. 2004;11(2):165–70.
- Kelly RF, Sluiter W, McFalls EO. Hibernating myocardium: is the program to survive a pathway to failure? Circ Res. 2008;102(1):3–5.
- Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288(3):984–99.
- Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998;97(18):1848–67.
- Tao ZW, Huang YW, Xia Q, Fu J, Zhao ZH, Lu X, et al. Early association of electrocardiogram alteration with infarct size and cardiac function after myocardial infarction. J Zhejiang Univ Sci. 2004;5(4):494–8.
- Pride YB, Giuseffi JL, Mohanavelu S, Harrigan CJ, Manning WJ, Gibson CM, et al. Relation between infarct size in ST-segment elevation myocardial infarction treated successfully by percutaneous coronary intervention and left ventricular ejection fraction three months after the infarct. Am J Cardiol. 2010;106(5):635–40.
- Delewi R, Ijff G, van de Hoef TP, Hirsch A, Robbers LF, Nijveldt R, et al. Pathological Q waves in myocardial infarction in patients treated by primary PCI. JACC Cardiovasc Imaging. 2013;6(3):324–31.
- Yang H, Pu M, Rodriguez D, Underwood D, Griffin BP, Kalahasti V, et al. Ischemic and viable myocardium in patients with non–Q-wave or Q-wave myocardial infarction and left ventricular dysfunction: a clinical study using positron emission tomography, echocardiography, and electrocardiography. J Am Coll Cardiol. 2004;43(4):592–8.
- Plein S, Younger J, Sparrow P, Ridgway J, Ball SG, Greenwood JP. Cardiovascular magnetic resonance of scar and ischemia burden early after acute ST elevation and non-ST elevation myocardial infarction. J Cardiovasc Magn Reson. 2008;10:47.
- Moon JC, Arenaza DP, Elkington AG, Taneja AK, John AS, Wang D, et al. The pathologic basis of Q-wave and non–Q-wave myocardial infarction. J Am Coll Cardiol. 2004;44(3):554–60.
- Cacciapuoti F. Echocardiographic evaluation of ejection fraction: 3DE versus 2DE and M-Mode. Heart Views. 2008;9(2):71–9.
- Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recomendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–119.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id