
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
The effect of moderate-intensity acute aerobic exercise duration on the percentage of circulating CD31+ cells in lymphocyte population
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i1.1277 Med J Indones. 2016;25:51–6
Received: August 29, 2015
Accepted: February 16, 2016
Author affiliation:
1 Department of Physiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Physiology, Faculty of Medicine, Atma Jaya Catholic University of Indonesia, Jakarta, Indonesia
3 Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Mariani Santosa
E-mail: niawidj@gmail.com
Background
The increasing number of circulating CD31+ endothelial progenitor cells is one of the important factors for maintaining vascular homeostasis. Exercise will effectively increase the number of circulating CD31+ endothelial progenitor cells. This study aims to determine the effect of moderate-intensity acute aerobic exercise duration on the percentage of circulating CD31+ cells in untrained healthy young adult subjects.
Methods
This study was an experimental study. Untrained healthy volunteers (n=20) performed ergocycle at moderateintensity (64–74% maximum heart rate) for 10 minutes or 30 minutes. Immediately before and 10 minutes after exercise, venous blood samples were drawn. The percentage of CD31+ cells in peripheral blood was analyzed using flow cytometry. Data was statistically analyzed using student t-test.
Results
There were no significant differences in the mean percentage of circulating CD31+ cells before and after exercise for 10 minutes and 30 minutes (p>0.05). However, there was a different trend in the percentage of circulating CD31+ cells after exercise for 10 minutes and 30 minutes. In the 10 minutes duration, 50% of subjects showed increase. Whereas in the 30 minutes duration, 80% of subjects showed increase.
Conclusion
The percentage of circulating CD31+ cells before and after exercise for 10 minutes was not different compared to 30 minutes. However, data analysis shows that majority of subjects (80%) had increased in the percentage of circulating CD31+ cells after 30 minutes exercise.
Keywords
CD31+ peripheral blood mononuclear cells, circulating endothelial progenitor cells, endothelial regeneration, exercise
Cardiovascular diseases has become a global health problem. Based on the report by WHO, in 2015 the number of deaths from cardiovascular diseases will increase to 20 million, about 30% of all deaths in the world.1 The WHO has estimated that if the major risk factors were eliminated, at least 80% of cardiovascular disease would be prevented.2 Therefore, promotion and preventive efforts should continue to be encouraged, prioritized, and reach all socioeconomic groups.
Endothelial dysfunction is associated with most forms of cardiovascular diseases.3 There are two mechanisms to replace the damaged endothelium. First, through the migration and proliferation of adjacent mature endothelial cells. Second, through alternative mechanism by endothelial progenitor cells. These cells are derived from bone marrow, circulate in the peripheral blood, and can differentiate into mature cells with endothelial characteristics.4 Endothelial progenitor cells contribute in maintaining vascular homeostasis through endothelial regeneration and neovascularization.5 Until now, there are no specific markers that could be used to identify human endothelial progenitor cells. Many researchers have identified circulating endothelial progenitor cells with flowcytometry using a single surface marker such as cluster of differentiation 34 (CD34), cluster of differentiation 133 (CD133), vascular endothelial growth factor receptor-2 (VEGFR-2) or various combinations of surface markers. One of the many surface markers that can be used for identifying endothelial progenitor cells is cluster of differentiation 31 (CD31).6 Based on a research conducted by Kim et al7, endothelial progenitor cells were almost exclusively confined to the CD31+ cell fraction, and culture of CD31+cells under defined conditions gave rise to endothelial cells.7 Recent studies provide evidence that exercise-either performed as a single session or training program-induced improvement in endothelial function. This is mainly due to mobilization of endothelial progenitor cells to peripheral circulation. Thus, exercise is an important method to promote cardiovascular health. Increased number of endothelial progenitor cells after exercise is probably caused by acute mobilization process of endothelial progenitor cells contained in bone marrow or due to shear stress that induces the release of endothelial progenitor cells into circulation.8,9
Given the important role of exercise for cardiovascular health, in 2007 the American College of Sports Medicine (ACSM) and the American Heart Association (AHA) recommend that all healthy adults need to do moderateintensity aerobic exercise for a minimum of 30 minutes (or can be accumulated toward the 30 minutes minimum by performing bouts each lasting 10 minutes) five days per week.10 However limited time available and low physical fitness often prevents a person to fulfill these recommendations, particularly in terms of duration. Therefore, accuracy in choosing the optimal duration of exercise becomes very important. Some studies related to the effect of exercise duration on the number of circulating endothelial progenitor cells in healthy individuals have been done with various results.8,11. The aim of this study is to investigate the effect of moderate-intensity acute aerobic exercise on the percentage of circulating CD31+ cells in untrained healthy individuals.
METHODS
Study subjects
Twenty healthy volunteers selected for this study were 20-22 years old male who never did regular physical activity [untrained, their maximum oxygen consumption (VO2max) correspond to this level of physical activity] and not on any medication known to interfere with the cardiovascular system. Subjects do not smoke or consume alcohol. Physical examination excluded apparent pathologies. Subjects were divided randomly into two groups: first group (10 subjects) performed ergocycle with moderate-intensity (64–74% maximum heart rate) for 10 minutes, and second group (10 subjects) performed ergocycle with moderate-intensity (64–74% maximum heart rate) for 30 minutes. The study protocol has been approved by the Research Ethics Commitee of the Faculty of Medicine, Universitas Indonesia (No. 824/UN2.F1/ETIK/2014). All subjects gave their written informed consent before participation.
Study design
This was an experimental study with selfcontrol design (before and after). The study was conducted in the Faculty of Medicine, Universitas Indonesia from December 2014 until February 2015. Experiments started in the morning (08:00 A.M). Subjects did not perform any strenous activities for at least 24 hours and were advised to have breakfast at least two hours before testing.
Determination of maximum oxygen consumption (VO2max)
Astrand six minute cycle test was conducted to validate that all subjects are untrained. Astrand protocol was performed on leg-cycling ergometer (Monark, Swedia) for six minutes.10 Suggested work rates for unconditioned males are 300 or 600 kg.m.min-1 (50 or 100 Watts). Pedal rate set at 50 rpm (use metronome). The goal is to obtain heart rate between 125 and 170 bpm. Heart rate during the fifth and sixth minutes of the test were measured. If these heart rates differ by more than ±5 bpm, the test is continued until steady-state heart rate is achieved. To estimate VO2max using this protocol, we use the nomogram. Once the VO2max has been determined, we multiply the VO2max value by the appropriate correction factor (based on age). Subject is called untrained if the value of VO2max is less than 45 mL/kg/min.12
Single exercise bout
Exercise protocols were applied based on the ACSM and AHA recommendations for apparently healthy adults with minimal physical activity (64–74% maximum heart rate for 10 minutes or 30 minutes).10 Single exercise bout was performed on leg-cycling ergometer (Monark, Swedia). Pedal rate set at 50 rpm (use metronome). Before the single exercise bout, subjects were seated in the upright position for at least 10 minutes before the baseline blood sample was drawn from antecubital vein. Subjects performed 10 minutes or 30 minutes ergocycle maintaining 64–74% of maximum heart rate (220-age) as aerobic exercise. Finally, 10 minutes after cessation of the cycling test, the post exercise blood sample was taken.
Flowcytometry
Venous blood was collected in Ethylene Diamine Tetra Acetic (EDTA) acid tubes and processed immediately. Anticoagulated blood was mixed with Roswell Park Memorial Institute (RPMI) medium at a ratio of 1:1, and the mixed sample was then layered on to Histopaque (Sigma- Aldrich, USA). Gradient centrifugation technique was performed to isolate mononuclear cells (MNCs). Flow cytometry analysis was performed with fluorescence activated cell sorting-calibur (FACS-Calibur) instrument (BD Biosciences, USA) and analyzed with CellQuestTM software (BD Biosciences, USA). One hundred microliters isolated MNCs were incubated with 10 μl Phycoerythrine (PE-) labeled Anti-human CD31 (BD Pharmingen, USA) at room temperature in the lab cabinet for 20 minutes. The lymphocyte population gate was adjusted to forward scatter (FCS) and side scatter (SSC) profiles of isolated MNCs (Figure 1). PE isotype matched antibody (BD Pharmingen, USA) was used to set the cut off point for CD31+ cells in the lymphocyte subpopulation. A total of 1x104 events were analyzed. The result was displayed as percentage of cells positive for CD31.
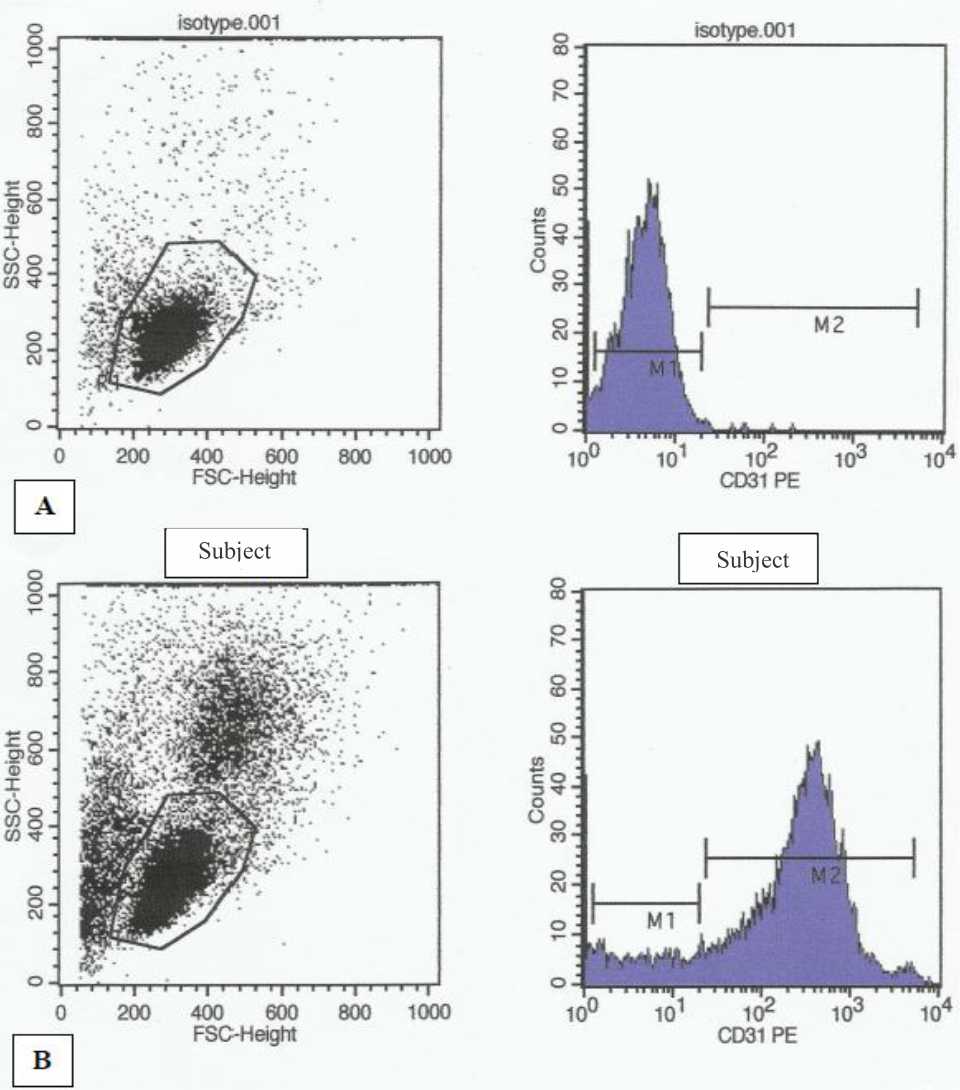
Figure 1. Flowcytometry analysis of CD31+ cells in peripheral blood. Mononuclear cells isolated from peripheral blood were gated in a forward-scatter (FSC)/side-scatter (SSC) plot: the cell population corresponding to small lymphocytes was gated and analyzed for background flourescence after labeling with isotype-matched control phyco erythrin (PE) mouse immunoglobulin G1 (second panel from left). (B) Flowcytometry analysis of CD31+ cells in one subject
Statistical analysis
The dependent and independent variables in this study were percentage of circulating CD31+ cells and exercise duration (10 and 30 minutes). All data analyses were performed using SPSS for Windows version 15.0 (SPSS Inc. Chicago, USA). Results are presented as mean ±SD. Data were examined for normal distribution using Saphiro Wilk test of normality. Comparisons among groups of study measures were done by student t-test (paired and unpaired). Probability values of p<0.05 were considered significant.
RESULTS
Characteristics of the subjects are summarized in table 1. Subjects in this study were healthy young adults, aged between 20–22 years old, has a normal body composition [mean body mass index (BMI) less than 25 kg.m-2, mean waist circumference less than 99 cm] and untrained. The average value of VO2max in all subjects is less than 38 mL/kg/min. This result showed that all subjects in this study are untrained. Percentage of circulating CD31+ cells before and after exercise in both groups (10 minutes vs 30 minutes) were assessed using student t test and are presented in table 2 and figure 2. Table 2 shows a decrease in the mean percentage of circulating CD31+ cells after 10 minutes exercise compared with before. While after 30 minutes of exercise, there is an increase in mean percentage of circulating CD31+ cells compared with before exercise.
Table 1. Characteristics of study subjects
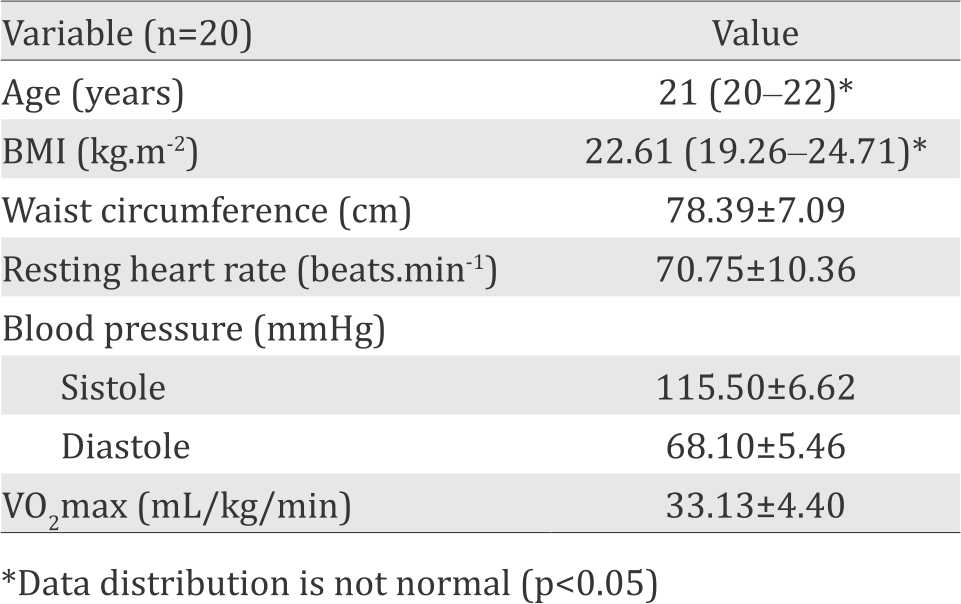
Table 2. Percentage of circulating CD31+ cells before and after exercise for 10 minutes and 30 minutes
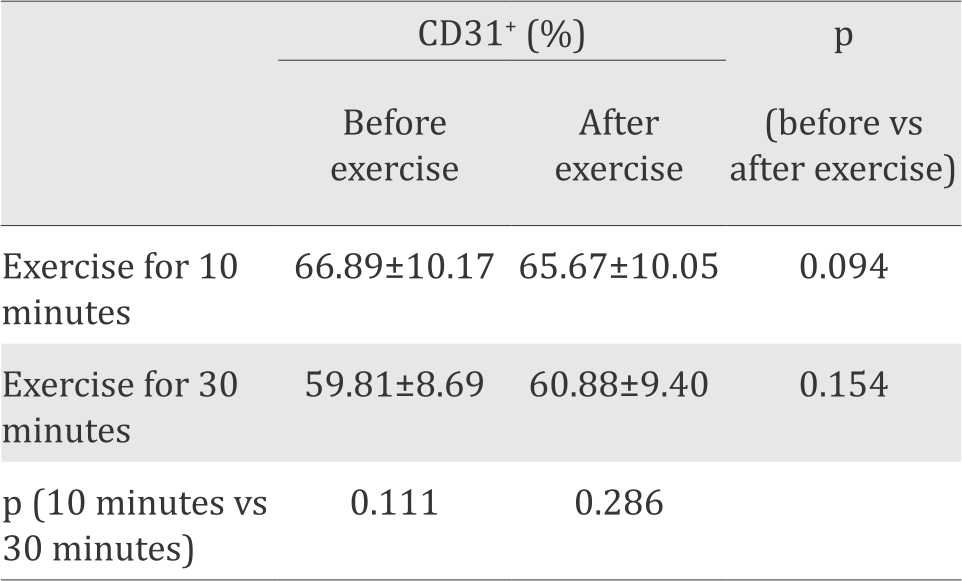
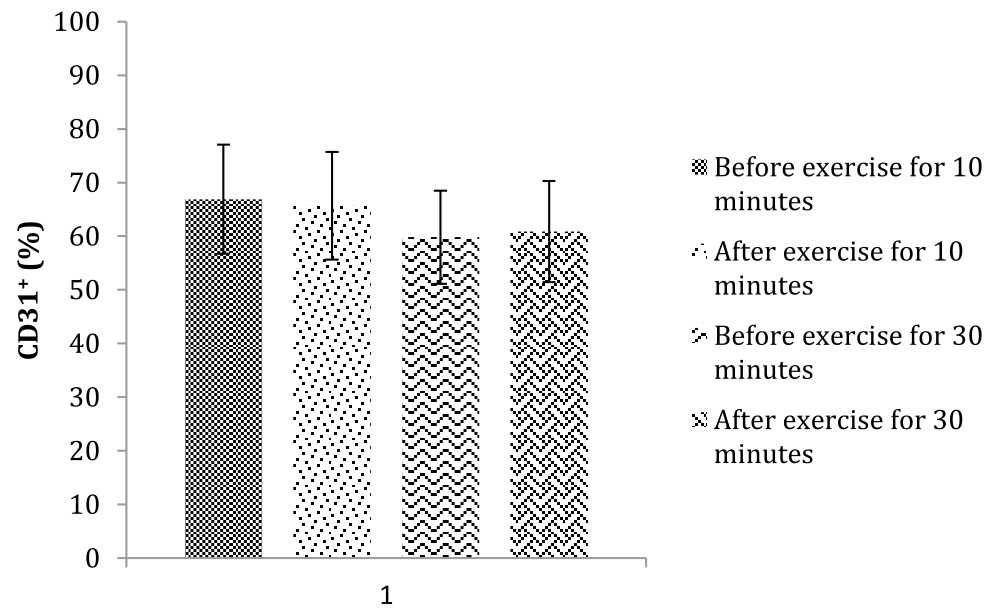
Figure 2. The mean percentage of circulating CD31+ cells before and after exercise for 10 minutes and 30 minutes
The different trend between effect of 10 minutes compared with 30 minutes exercise on percentage of CD31+ endothelial progenitor cells is described further in figure 3. After 10 minutes exercise, 50% of subjects (five subjects) showed increase (0.3%–0.8% of the value before exercise) and 50% of subjects (five subjects) showed decrease (1.7%–4.8% of the value before exercise) in the percentage of circulating CD31+ cells after exercise. However, the increment showed a relatively small number. After 30 minutes exercise, 80% of subjects (eight subjects) showed increase (0.1%–4.2% of the value before the exercise) and 20% of subjects (two subjects) showed decrease (1.3%–2.7% of the value before exercise) in the percentage of circulating CD31+ cells after exercise.
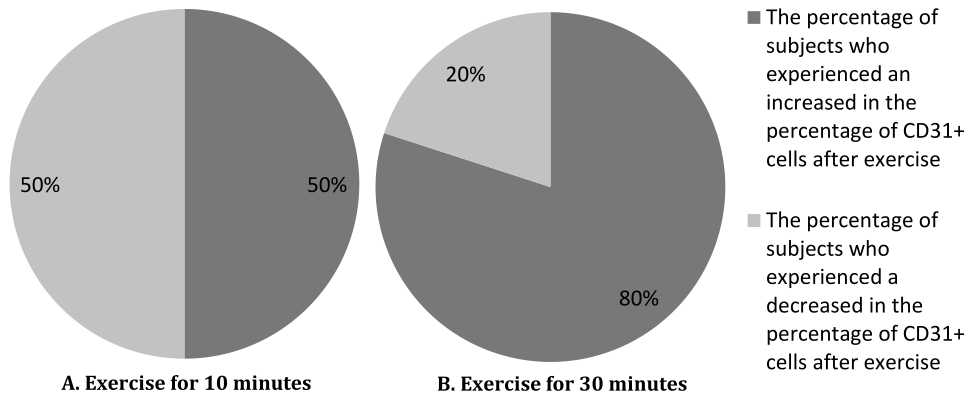
Figure 3. Pie diagram showing the percentage of subjects who experienced the increase and the decrease in the percentage of circulating CD31+ cells after exercise for 10 minutes and 30 minutes
DISCUSSION
CD31 is a surface marker found on mature endothelial progenitor cells.6,13 Other cells in the peripheral blood that express CD31 surface marker are monocytes, granulocytes and platelets.7 Flow cytometry analysis of CD31+ cells in this study was performed in the lymphocyte sub-population from isolated MNCs. The gating strategy was specifically selected to sub-population of MNCs from peripheral blood with the highest probability of identifying endothelial progenitor cells.
Our results showed higher percentage of circulating CD31+ cells than previous studies. This could be caused by different gating strategy in flow cytometry analysis. We used only CD31 staining to identify endothelial progenitor cells, whereas phenotypic of circulating endothelial progenitor cells are based on coexpression of endothelial and hematopoietic antigens such as cluster of differentiation 34/ vascular endothelial growth factor receptor-2/ cluster of differentiation 31/vascular endothelialcadherin (CD34/VEGFR-2/CD31/VE-cadherin). Therefore this becomes a limitation of this study.
These results are consistent with study conducted by Laufs et al14, who demonstrated that moderateintensity aerobic exercise for 10 minutes in healthy young adults did not increase the number of circulating endothelial progenitor cells.14 In the study conducted by Laufs et al14 there is an increase in the average number of endothelial progenitor cells that are different from the results of this study (the increase varies, 50% of subjects experienced an increase with a value below 1% and 50% of subjects experienced a decrease). This could be due to differences in exercise protocol and marker used to identify a population of endothelial progenitor cells.
On the other hand, these results are in contrast to studies conducted by Laufs et al14 and Cubbon et al,15 who demonstrated that moderate-intensity aerobic exercise for 30 minutes in healthy young adults can significantly increase the number of circulating endothelial progenitor cells.14,15 This could be due to differences in the type of exercise, intensity of exercise, marker(s) used for identification of endothelial progenitor cells, and genetic or characteristics of the subject.
The type of aerobic exercise in a study by Laufs et al14 was running. According to ACSM, running is a kind of strenuous physical activity and causes an increase in heart rate higher than cycling. It will affect the physiological adaptation process of vascular system to exercise because this process is specific to the type of exercise performed.10
Exercise intensity used in Laufs et al14 and Cubbon et al15 studies are much higher than this study, which is about 68% VO2max or equal to 80% maximum heart rate.16 We assume that the intensity of exercise used in our study may not be enough to generate similar response as the study by Laufs et al14 and Cubbon et al.15
In Laufs et al14 and Cubbon et al15 studies, they used a combination of markers such as CD34+ / VEGFR-2+/CD133+/CD177+/CD45- to identify immature endothelial progenitor cells. Whereas in this study, we used CD31 single marker. Immature endothelial progenitor cells will differentiate into mature endothelial progenitor cells marked by expression of CD31.13 In this study, differences in the pattern of change in the percentage of CD31+ cells after exercise for 30 minutes compared to 10 minutes may be due to migration of immature endothelial progenitor cells from the bone marrow into peripheral blood and maturation process in the circulation.
Genetic background e.g race also showed differences in percentage of circulating endothelial progenitor cells after exercise. Asians according to a study by Cubbon et al15 showed slight increase in percentage of endothelial progenitor cells after exercise when compared with Europeans.15 This is in line with the result from this study. Other differences in subject characteristics which influence the outcome of this study when compared with Laufs et al14 study was the level of training in study subjects. Trained subjects as in Laufs et al14 study are known to have higher endothelial progenitor cells basal values which may significantly affect percentage increment after exercise. This study used untrained subjects therefore had lower basal values as well as insignificant increment were obtained.9
However, from the results of this study, we can see there is a pattern of an increase in the percentage of circulating CD31+ cells after 30 minutes exercise. Changes in the percentage of CD31+ cells after exercise for 30 minutes on each subject varied. Where 80% of subjects (eight subjects) showed increase (0.1%–4.2% of the value before the exercise) and 20% of subjects (two subjects) showed decrease (1.3%–2.7% of the value before exercise) in the percentage of circulating CD31+ cells after exercise. Variations of this value is probably due to endothelial nitric oxide synthase (eNOS) enzyme polymorphism. eNOS enzyme influence mobilization of immature endothelial progenitor cells from the bone marrow or in other words a normal vascular adaptation in basal condition. Individuals with eNOS polymorphism would demonstrate less vascular adaptation at the time of exercise, hence the variation occurs.17 Further investigation is needed to confirm our finding.
The mechanism by which exercise induces increase in the number or percentage of circulating endothelial progenitor cells is through mobilization from the bone marrow. Two determining factors by which exercise mobilize higher or lower magnitude of endothelial progenitor cells are exercise intensity and duration. Exercise with moderate or high-intensity (lasting less than one hour) cause a lower magnitude increase in the number of circulating endothelial progenitor cells, and their main mechanism of mobilization seems related to nitric oxide bioavailability. Increased bioavailability of nitric oxide (NO) will cause the activation of metalloproteinase nine and the release of soluble kit ligand, that would lead to mobilization of endothelial progenitor cells from bone marrow to circulation. On the other hand, long or ultralong duration exercise can generate mobilization response with a higher number and has a longer lasting effect and this is closely related to plasma VEGF levels, the most effective mobilizer for endothelial progenitor cells.11,18
In conclusion, the percentage of circulating CD31+ cells before and after exercise for 10 minutes was not different compared to 30 minutes. Although not statistically significant, there is a pattern of an increase in the percentage of circulating CD31+ cells after 30 minutes exercise. Majority of subjects (80%) had increased in the percentage of circulating CD31+ cells after 30 minutes exercise.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
None.
REFERENCES
- Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease. Promoting cardiovascular health in the developing world: a critical challenge to achive global health. Fuster V, Kelly BB, editors. Washington (DC): National Academies Press (US); 2010. p. 49.
- World Health Organization. Preventing chronic disease: a vital investment. Geneva: World Health Organization; 2005. p. 15.
- Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–69.
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–95.
- De Biase C, De Rosa R, Luciano R, De Luca S, Capuano E, Trimarco B, et al. Effects of physical activity on endothelial progenitor cells (EPCs). Front Physiol. 2013;4:414.
- Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined?. J Cell Mol Med. 2009;13(1):87–102.
- Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;10(5):602–14.
- Koutroumpi M, Dimopoulos S, Psarra K, Kyprianou T, Nanas S. Circulating endothelial and progenitor cells: evidence from acute and long term exercise effects. World J Cardiol. 2012;4(12):312–26.
- Siddique A, Shantsila E, Lip GYH, Varma C. Endothelial progenitor cells: what use for the cardiologist?. J Angiogenes Res. 2010;2(6):1–13.
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8th ed. Philadelphia: Lippincott Wiliams & Wilkins; 2010. p. 7–76.
- Silva JF, Rocha NG, Nóbrega AC. Mobilization of endothelial progenitor cells with exercise in healthy individuals: a systematic review. Arq Bras Cardiol. 2012;98(2):182–91.
- Sharkey BJ. Physiology of fitness. 3rd ed. USA: Human Kinetics Publishers; 1990. p. 19.
- Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23(7):1185–9.
- Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, et al. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12(4):407–14.
- Cubbon RM, Murgatroyd SR, Ferguson C, Bowen TS, Rakobowchuk M, Baliga V, et al. Human exerciseinduced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol. 2010;30(4):878–84.
- Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc. 1994;26:112–6.
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy?. Cardiovasc Res. 2005;67(2):187–97.
- Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, et al. Hemopoietic and angiogenic progenitors in healthy athletes: different responses to endurance and maximal exercise. J Appl Physiol (1985). 2010;109(1):60–7.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id