
Section Abstract Introduction Case Illustration Discussion Conflict of Interest Acknowledgment References
Case Report
A tale of the broken heart: peripartum cardiomyopathy, a case report
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i1.1278 Med J Indones. 2016;25:57–61
Received: August 29, 2015
Accepted: January 15, 2016
Author affiliation:
1 Department of Anesthesiology and Critical Care Medicine, Siloam Hospitals Lippo Village, Tangerang, Indonesia
2 Department of Anesthesiology, Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Indonesia
Corresponding author:
Hori Hariyanto
E-mail: horimd@yahoo.com
Progressive dyspnea following childbirth warrants a prompt suspicion into the diagnosis of peripartum cardiomyopathy, PPCM. Pump failure causes an inadequate cardiac output which ultimately contributes to PPCM high mortality rate; however early airway control, vigilant fluid balance and vasoactive support will substantially reduce the incidence of patients falling into decompensated heart failure. More importantly, it is imperative that these patients are cared in a setting where continuous hemodynamic monitoring is available. This case report serves as a reminder not to focus end-point therapy solely on blood pressure readings, but to observe signs and symptoms of hypoperfusion such as cold clammy skin, cool extremities, decreased urine output and mental status.
Keywords
acute heart failure, management, peripartum
Peripartum cardiomyopathy (PPCM) is a rare and challenging situation once encountered in the clinical setting. Within the obstetric population, dilated cardiomyopathy (DCM) is the most common form of myopathy seen and involves mild to severe left ventricular systolic dysfunction. Risk factors include obesity, multiparity, advanced maternal age (>30 years), multifetal pregnancy and preeclampsia. However the number one cause of PPCM is viral myocarditis.1
Peripartum cardiomyopathy has a low incidence affecting 0.1% of pregnancies, but it carries a high morbidity and mortality from 7% to 50% and has variable outcomes.2 The clinical signs and symptoms are dyspnea, orthopnea, and paroxysmal nocturnal dyspnea due to increased ventricular pressure and subsequent fluid accumulation from the over-congested left ventricle. If left untreated, cardiomyopathies will lead to multiple organ failure due to the inability of the heart to fill with or eject blood at a rate appropriate to meet tissue requirements.3
Therefore, the cornerstone of successful management in such a case requires prompt airway and breathing control, vigilant fluid balance coupled with the use of balanced vasoactive drugs. In this case report, we present the management of acute heart failure due to peripartum cardiomyopathy in the intensive care unit.
CASE ILLUSTRATION
A 27 years old female with para 2, live 1, abortus 1 (P2L1A1) presented to the intensive care unit (ICU) after experiencing progressive worsening dyspnea 12 hours after an emergency C-section due to a nuchal cord, under spinal anesthesia. The patient had no prior history of tocolytic usage, coagulation abnormalities, recent infection or cardiac abnormalities. Routine antenatal care did not reveal the presence of hypertension and/or pre-eclampsia, but her weight had significantly increased 30 kg throughout the pregnancy. In the general ward, our patient began feeling short of breath which continued to worsen over two hours despite applying a simple mask at 10 LPM oxygen. Her saturation remained below 80% with a heart rate of 150–165 bpm, blood pressure of 140/85 mmHg, and respiratory rate of 40 per minute. Arterial blood gas revealed respiratory acidosis: pH 6.9, PaO2 161 mmHg, PaCO2 78.6 mmHg, HCO3 -14.7 mmol/L, BE -20.7 mmol/L. Physical examination revealed diffuse rales on both lungs, cold and clammy extremities and she was agitated. An initial diagnosis of acute lung edema was made.
The patient was rushed to the ICU where she was immediately intubated using intravenous 5 mg midazolam and placed on mechanical ventilation with morphine: midazolam sedation. Ventilatory support was set to pressure control mode with peak end expiratory pressure (PEEP) 10, respiratory rate 20, and FiO2 of 0.6. The right subclavian vein was cannulated as a central access and meropenem was given on the first and second day, subsequently. Within one hour of admission, her blood pressure dropped to 70/50 mmHg with a heart rate of 170 bpm, despite 200 mL of fluid challenge. Norepinephrine at 0.1 μg/kg/min, 3 μg/kg/min dobutamine, 5 μg/ min of nitroglycerin infusion, and 5 mg/hour of furosemide infusion were started.
Chest X-ray revealed cardiomegaly with a cardiothoracic ratio of 63%, pulmonary edema and bilateral pleural effusion (Figure 1). Continuous electrocardiography monitoring showed sinus tachycardia with no ST segment or T wave changes. Hematologic results were within normal values except for white cell count of 18,350 /mm3 (N: 8,000-15,000 /mm3), Hb 11.68 g/dl, troponin T <0.01 ng/mL, C-reactive protein 0.21 mg/dl (N: <3), lactic acid 1.6 mg/dL (N: <4.0) and procalcitonin 0.19 ng/mL (N: <0.15). An echocardiogram revealed a severely impaired left ventricular systolic function with an ejection fraction (EF) of 19% (N: ≥55%) with severely hypokinetic infero-posterior ventricle wall region. On day one, diuresis was noted to be 100 mL/hour and the fluid balance was +605 mL/12hours.
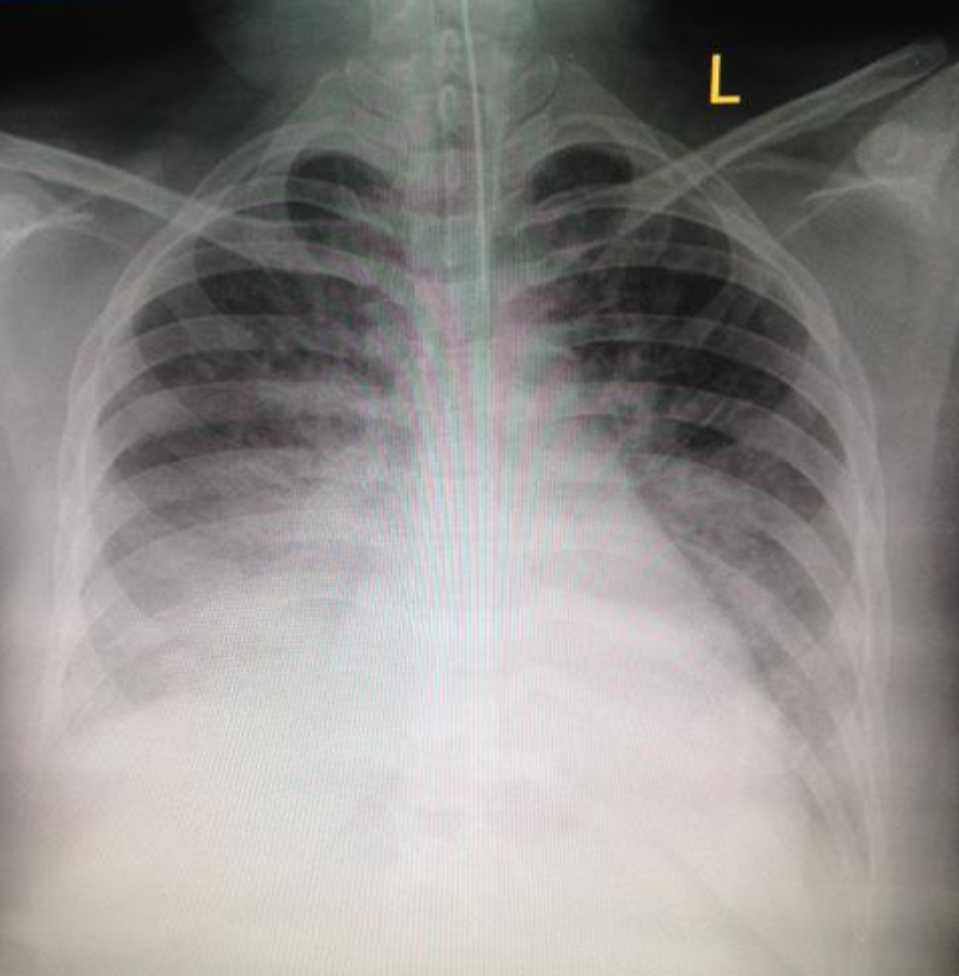
Figure 1. Chest X-ray on day one, one hour after intubation
On day two, her ventilatory support was weaned to PEEP 5, respiratory rate 15, and FiO2 was decreased to 0.35. An arterial blood gas response showed pH 7.386, pO2 190.8, pCO2 32.3, HCO3- 19.5 and BE of -4.1 mmol/L. Norepinephrine infusion was tempered down to 0.03 μg/kg/min, 3 μg/kg/min dobutamine, 5 μg/min of nitroglycerin infusion, and 5 mg/ hour of furosemide infusion. Her blood pressure was maintained at 130/80 with a heart rate of 130 bpm. Normal saline was infused at 10 mL/ hour and diuresis was noted to be 234 mL/hour. Her fluid balance was -5079 mL/24 hours. Her mentation greatly improved and she was able to respond appropriately.
On day three, norepinephrine was discontinued and her only support were 2.5 μg/kg/min dobutamine, 5 μg/min of nitroglycerin infusion, and 5 mg/hour of Lasix infusion. She was extubated shortly thereafter and all support was completely discontinued 12 hours postextubation. Her oral fluid intake was restricted to 1000 mL/24 hours, diuresis was noted to be 120 mL/hour and the fluid balance was -2473 mL/24 hours. Her medication at this time was 20 mg furosemide tablet, daily. Vital parameters remained stable and she remained alert.
A follow-up chest X-ray on day four showed marked reduction in the amount of infiltrates on both lung regions. Her cardiothoracic ratio measured 60% (Figure 2). Diuresis was noted to be 105 mL/hour and the fluid balance was -2517 mL/24 hours. She continued taking daily 20 mg furosemide tablet. Aside from vasoactive medications and strict fluid control, it should be noted that our patient also received sedation and daily subcutaneous heparin of 5,000 IU in order to decrease the risk of thromboembolism throughout her four days in the ICU.
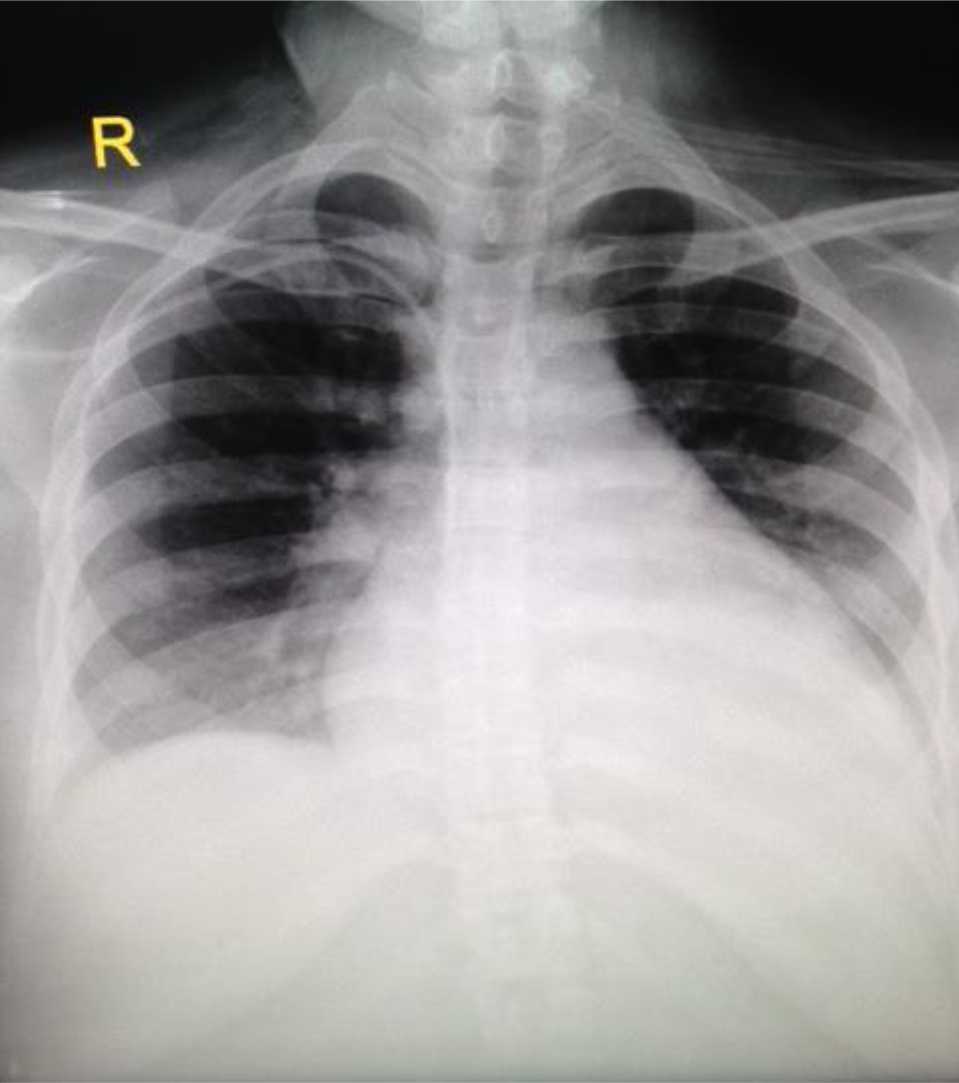
Figure 2. Chest X-ray on day three, eight hours after extubation
She was transferred to the general ward on day five and discharged from the hospital on day 17 with oral furosemide 40 mg/day, candesartan 2 mg/day, potassium tablets 600 mg/day, and trimetazidine 35 mg/day. A follow-up echocardiography prior to discharge was not available due to financial problems. However, a follow-up visit one week after discharge revealed her vital signs: Blood Pressure 120/80 mmHg; Heart rate 95 beats per minute, sinus rhythm, respiratory rate 14 breaths per minute, no pedal edema, and she has continued her daily activities without any dyspnea noted.
DISCUSSION
In this case report, we see a non-classic clinical presentation of acute pump failure in a healthy patient with no prior signs of cardiac abnormalities. Our patient reported a pregnancy weight gain of 30 kg indicating that excessive fluid retention has taken place, as the expected average weight gain during pregnancy is 14.5 kg. Any additional increase from the average weight is commonly due to fluid retention.4 After delivery occurred, she started feeling dyspneic due to the sudden increase in venous return after intense uterine contraction and involution. As a result, heart failure may follow due to excessive end diastolic volume and failure of ventricular compliance to eject the excessive blood.
In this patient, PPCM was diagnosed on the basis of acute progressive orthopnea and dyspnea and dilated dysfunctional left ventricle upon echocardiography within 24 hours of delivery. Diffuse rales upon chest auscultation and chest X-ray showing pulmonary edema, bilateral pleural effusion and cardiomegaly strengthen the diagnosis of acute pump failure leading to signs of fluid overload. Her echocardiographic results of an EF less than 45% occurring within five months postpartum without any prior history of cardiac or valve dysfunction confirm the diagnosis of cardiomyopathy.5 A normal electrocardiography and normal troponin level exclude the diagnosis of myocardial infarction and myocarditis.
Interstitial pulmonary edema causes persistent hypoxemia as shown by her oxygen saturation that remained at 80% using a 10 LPM oxygen flow by simple face mask. The pathophysiology stems from pulmonary interstitial space, a region between the alveolar epithelium and capillary epithelium where gas exchange could only take place. If this region is filled with excessive fluid from an over congested left ventricle, the space widens and gas exchange becomes disrupted.6 Intubation and mechanical ventilation were decided as early as possible as persistent hypoxic condition is a well-known cause of pulmonary vasoconstriction. If left untreated, it will result in increased right ventricle pressure and put further stress on the already “strained” heart. Analgesia and sedation with morphine and midazolam was given to reduce oxygen consumption and aid in smooth mechanical breathing.
In cardiogenic edema, a high initial PEEP was given in order to open and recruit more alveoli. By using PEEP, the alveoli are kept in a distended manner and increase the surface area available for gas exchange.7 In this patient, a calculated alveolararterial (A-a) gradient was 454 mmHg (normal value ≤15 mmHg) and a PaO2/FiO2 ratio of 268 which are the combination of interstitial edema and low cardiac output. Systolic dysfunction causes shunting of pulmonary blood to the circulation. An increased heart rate, increased blood pressure with diffuse rales and dyspnea are clear indications that systemic circulation was halted at the ‘pump’ level.
Her echocardiography revealed normal sized heart chambers; however the left ventricle ejection fraction was severely impaired. A severely hypokinetic infero-posterior region on the heart will significantly affect myocardial contractility, hence impairing cardiac output.8,9 For this reason, inotropic and vasoactive medications are needed as a crucial support in the management of acute systolic dysfunction.10 Loop diuretics are necessary to reduce preload while nitroglycerin is utilized to dilate arterial smooth muscles and reduce the “pump” workload.
Twenty four hours after initiating mechanical ventilation, inotropy support and diuretics the alveolar-arterial (A-a) gradient became 54.1 and her PaO2/FiO2 index improved significantly to 545. Such value reflects an improving oxygenation status and confirms the disturbance of gas exchange from a cardiac origin. Fluid balance was deliberately strived to negative in order to reduce fluid overload while carefully titrating to the patient’s hydration status and mentation.10 Intravenous antibiotic was administered as a prophylaxis for her high initial white blood cell count and history of recent operation. Recovery from PPCM occurs between three and six months postpartum, but has been reported to occur as late as 48 months postpartum, with a mean ejection fraction (EF) of 0.28 to 0.46. Specifically, it is characterized by left ventricular ejection fraction (LVEF) recovery to ≥0.50 or improvement by >0.20.11,12 Our patient has returned for a one week follow-up visit with normal vital parameters and no complain of dyspnea with daily activities. She is due for a follow-up echocardiography, 40 days postpartum.
In conclusion, the management of acute heart failure due to cardiomyopathy must be established and promptly treated. Airway must be secured early because interstitial fluid accumulation results in airway edema and may worsen hypoxemia from the low cardiac output during “pump failure”. Next critical care interventions must be directed at 1) initiating mechanical ventilation with sedation to optimize oxygen delivery, 2) administering positive inotropic and vasoactive drugs to increase contractility and maintain an optimum mean arterial pressure (MAP) for organ perfusion, 3) reducing preload and afterload to reduce left ventricular workload, and 4) fluid balance is kept negative while keeping close attention to adequate perfusion signs such as improving mental status, warm extremities and adequate urine output.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
We certify that this report is our own work and that all sources of information used in this report have been fully acknowledged.
REFERENCES
- Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–93.
- Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, Hsu JW, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100(2):302–4.
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: national heart, lung, and blood institute and office of rare diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183–8.
- Conrad KP, Karumanchi SA Renal physiology and diseases in pregnancy. 5th ed. In: Alpern RJ, Caplan MJ, Moe OW, editors. Seldin and Giebisch’s The Kidney: Physiology and Pathophysiology. United Kingdom: Elsevier; 2013. p. 2701–17.
- Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94(2):311–6.
- Wiener-Kronish JP, Broaddus VC. Interrelationship of pleural and pulmonary interstitial liquid. Annu Rev Physiol. 1993;55:209–26.
- Wiesen J, Ornstein M, Tonelli AR, Menon V, Ashton RW. State of evidence: mechanical ventilation with PEEP in patients with cardiogenic shock. Heart. 2013;99(24):1812–7.
- Harvey PA, Leinwand LA. Celular mechanisms of cardiomyopathy. J Cell Biol Dis. 2011;194(3):355–65.
- Zhang YH, Youm JB, Sung HK, Lee SH, Ryu SY, Lee SH, et al. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J Physiol. 2000;523(Pt3):607–19.
- Yancy CW, Jessup M, Butler J, Drazner MH, Geraci SA, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundataion/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–327.
- Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344(21):1567–71.
- Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–5.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id