
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Altered expressions of endothelial junction protein of placental capillaries in premature infants with intraventricular hemorrhage
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i3.1287 Med J Indones. 2016;25:143–50
Received: September 14, 2015
Accepted: August 25, 2016
Author affiliation:
1 Biomedical Sciences Doctoral Program Study, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Child Health, dr. Suyoto Hospital, Jakarta, Indonesia
3 Center of Hypoxia and Oxydative Stress Study, Departement of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
5 Department of Child Health, Budi Kemuliaan Women and Children Hospital, Jakarta, Indonesia
6 Department of Biochemistry and Biology Molecular, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Maria Ekawati
E-mail: mariaeka_harjadi@yahoo.co.id
Background
Placental hypoxia may lead to oxidative stress, which inflicts damage to capillary protein junction. The aim of this study was to evaluate altered expression of endothelial junction protein of capillaries in hypoxia condition and to observe its correlation with the incidence of intraventricular hemorrhage in premature infants.
Methods
A cross-sectional study was conducted by using placental tissues of premature infants as amodel of capillary integrity (29 hypoxic and 29 non-hypoxic). Hypoxia inducible factor (HIF)-1α was measured to define placental tissue response to hypoxia; malondialdehyde (MDA) and glutathione (GSH) served as markers of oxidative stress. The expressions of junctional proteins, N-cadherin and occludin were analyzed by immunohistochemistry. Intraventricular hemorrhage (IVH) was detected by cranial ultrasound at the third day. Unpaired t test, Mann-Whitney, and Chi-square tests were used to analyze the data.
Results
The HIF-1α and MDA levels were slightly, but not significantly, higher in hypoxia group {13.64±8.70 pg/mg protein and 10.31 pmol/mg tissue (ranged 1.92–93.61), respectively} compared to non- hypoxia group {10.65±5.35 pg/mg protein and 9.77 pmol/mg tissue (ranged 2.42–93.31)}. GSH levels were not different in both groups (38.14 (ranged 9.44–118.91) and 38.47(ranged 16.49–126.76) ng/mg protein, respectively. mRNA expression of N-cadherin (0.13) and occludin (0.096) were significantly lower in hypoxia comparedto non-hypoxia group (p=0,001), while protein expression of N-cadherin (3.4; 75.9; 6.9; 13.8%) and occludin (20.7; 3.4; 69.0; 3.4; 6.9%) in hypoxia group was not associated with IVH (p=0.783 and p=0.743).
Conclusion
Hypoxia altered expression of endothelial junction protein in placental capillaries, but no association with intraventricular hemorrhage was observed.
Keywords
endothelial junction protein, intraventricular hemorrhage, hypoxia, oxidative stress
Placenta is a multifunctional organ delivering nutrition and oxygen needed by the fetus through maternofetal circulation by passing the maternofetal barrier.1 The barrier, contains several layers including the syncytiotrophoblast, cytotrophoblast, connective tissue derived from mesoderm, and a single layer of capillary endothelium.2,3 The capillary endothelial layers consist of endothelial cells connected each other by transmembrane junction proteins.4 There are two areas of junction, i.e. the tight junction and adherent junction. Occludin is one of proteins found in the tight junction, while N-cadherin is a protein contained in the adherent junction.4,5 Those endothelial junction proteins have a function to control the permeability of endothelial layer.4,6,7
Several conditions such as hypoxia may cause damage on endothelial junction. Hypoxia causes imbalance between oxidants and antioxidants known as the oxidative stress,8,9 which damages lipid, nucleic acids, protein, DNA and other cellular components, including the intercellular junction protein in the capillary endothelium.8,10–12 Any damage of endothelial junction protein will result in the development of intercellular endothelial gap that may lead to increased permeability of capillary endothelial layer and disrupts capillary function.4,13
A study by Trollmann et al14 suggested that placenta hypoxia may indicate cerebral hypoxia distress in fetal rats, while another study evaluating hypoxia in premature infants found that cerebral hypoxia may become a predictor of the development of intraventricular hemorrhage.15 Intraventricular hemorrhage is a distinctive intracranial bleeding in premature infants caused by the increase in endothelial capillary permeability in the germinal matrix,16–18 an area in the brain which is only present in premature infants with gestational age of ≤36 weeks.5,16,19 The germinal matrix consists of a lot of capillaries containing a single endothelial layer. Just like maternofetal barrier in placenta, the blood brain barrier is constructed by capillary endothelial layers together with basal membrane containing pericytes, and perivascular astrocyte foot process.5,17
Data from Harapan Kita Woman and Children Hospital, Jakarta, 2012 demonstrated that one third of premature newborn suffered from intraventricular hemorrhage, and one third have hypoxia.
The aim of our study was to evaluate altered expression of endothelial junction protein of capillaries in hypoxia condition in premature infants and to observe its correlation with permeability changes, as well as the occurrence of intraventricular hemorrhage in premature infants. Due to limitation in obtaining brain specimens of premature infants, our study used placenta tissues as specimens of our samples.
METHODS
This was an observational cross-sectional study, conducted at the Molecular Laboratory for Oxidative Stress Studies, Department of Biochemistry; Molecular Biology Laboratory of Department of Physiology; and Department of Histology Faculty of Medicine, Universitas Indonesia, between January 2013 and December 2014.
The samples derived from placental tissue specimens obtained from premature infant born at Budi Kemuliaan and Harapan Kita Woman and Children Hospitals, Jakarta. The inclusion criteria were placental tissues of premature infants with gestational age of ≤36 weeks.
Specimens were grouped according to hypoxia (venous oxygen saturation <60%) and nonhypoxia group (venous oxygen saturation >60%). Placental tissues of infants with congenital defects were excluded.
Sample size was calculated using two proportions hypothesis test for two independent groups.
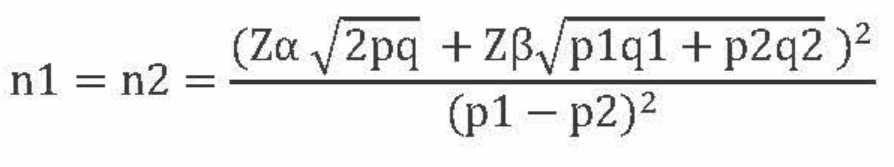
By taking p1 value 0.67 and p2 value 0.33, and the power β of 80%, a minimum of 33 samples were needed in each group.
Placental hypoxia was determined based on umbilical vein saturation measured by blood gas analysis.20 Intraventricular hemorrhage (IVH) was detected using cranial ultrasound on the third day. The protocol of the study has been approved by The Research Ethic Committee of the Faculty of Medicine, Universitas Indonesia (No.16/H2.F/ ETIK/2013).
ELISA technique for detecting HIF-1α protein level
Hypoxia inducible factor (HIF-1α) was measured using an enzyme-linked immunosorbent assay (ELISA) kit Cusabio. The microplate provided in this kit was pre-coated with an antibody spesific for HIF-1α. Standards or samples were added to microplate wells, added with a biotin-conjugated antibody preparation spesific for HIF-1α. Subsequently, horseradish peroxidase (HRP)-avidin was added to each microplate well followed by tetramethyl benzidine (TMB) substrate solution. Only the wells that contained HIF-1α, biotin-conjugated antibody, and enzyme-conjugated avidin would exhibit the change in color. The enzyme-substrate reaction was terminated by addition of stop solution, and color change was measured by ELISA reader at λ of 450 nm.
Wills method of placental MDA measurement
About 100mg of placental tissue in 1 ml of PBS at pH 7.0 was homogenized using micropestel and microtube. Subsequently, 200 μL of 20% trichloro acetic acid (TCA) was added into 50 μL of placental homogenate to induce protein precipitation and then treated with centrifugation with the speed of 5,000 g for 10 minutes. Supernatant was removed and 400 μL thiobarbituric acid (TBA) was added. After boiling for 10 minutes in water bath, the absorbance was measured at λ 530 nm.21
Ellman method of placental GSH measurement
Gluthation peroxidase (GSH) level was measured from 50 mL of placental homogenate in phosphate buffer at pH 8.0 which was added with 200 μL of 5% TCA for protein precipitation. After centrifugation with 5,000 g for 10 minutes, 25 mL of dithiobisnitrobenzoate (DTNB) solution was added to the supernatant of placental homogenate and incubated for one hour. Absorbance was measured at l = 412 nm. The level of GSH was measured as μg GSH/mg placental protein. The level of placental protein was calculated using the curve of standard bovine serum albumin (BSA) solution.22
Histomorphology of placental vascular tissue
Placental tissue was immersed in cold 0.9% NaCl, and cut in 3-5 mm thickness. After fixation, dehydration, clearing and embedding, then it was cut into sections with microtome in 4-5 μm thickness. Hematoxylin eosin staining was used for histomorphology analysis and the specimens were observed under light microscope. Evaluation was performed using modification of Moenadjat24 scoring system with a score of 1-3. Score 1: nuclei are not located in a single line, endothelial layer is not intact. Score 2: nuclei are located in a single line, endothelial layer is not intact, and score 3: nuclei are located in a single line, intact endothelial layer.23
Immunohistochemistry of N-cadherin and occludin detection
We performed deparafinization, dehydration, washing, addition of peroxidase blocking solution, washing, then incubate by N-cadherin and occludin primary antibodies with the ratio of 1:25. Incubation was continued by adding secondary antibody (novolink polymer). 3,3’-diaminobenzidine (DAB) solution was added to the specimen and then counterstained by incubating them in hematoxylin solution. The specimens were observed under light microscope. This assay examined the distribution and color intensity of the fine granules of N-cadherin and occludin. Evaluation was performed using modification of Moenadjat scoring with a score of 1-5.24
According to the Moenadjat24 modification scoring, score 1: the distribution of N-cadherin/ occludin molecule did not show color intensity. Score 2: the distribution of N-cadherin/occludin molecule was not homogenous on the wall, or cytoplasm of endothelium attached to basal membrane with moderate or weak intensity. Score 3: the distribution of N-cadherin/occludin molecule was homogenous on the wall, or cytoplasm of endothelium attached to basal membrane with moderate or weak intensity. Score 4: the distribution of N-cadherin/occludin molecule was not homogenous on the wall, or cytoplasm of endothelium attached to basal membrane with strong intensity. Score 5: the distribution of N-cadherin/occludin molecule was homogenous on the wall, or cytoplasm of endothelium attached to basal membrane with strong intensity.
RNA isolation and RT-qPCR technique for detecting mRNA of N-cadherin and occludin
Total mRNA samples were obtained from isolated placental tissue using the Rneasy mini kit (Qiagen). RNA concentration was determined using a NanoDrop 2000 (ThermoScientific). Real-time reverse transcriptase –q polymerase chain reaction (RT-qPCR) was performed using Rotor Gene Q-5plex (Qiagen) with QuantiTect SYBR Green RT PCR kit (Qiagen) one step method. rRNA 18S gene was used as internal control. The sequence of N-cadherin and occludin and 18S were obtained from National Center for Biotechnology Information (NCBI)-Gene bank. The primers of N-cadherin: forward 5’-TTCGGGTAATCCTCCCAAATC-3’, reverse 5’-CACAATCCTGTCCACATCT-3’, product 98 bp. The primers of occludin: forward 5’-TAACTTCGCCTGTGGATGAC-3’, reverse 5’-CTCTTTGACCTTCCTGCTCTT-3’, product 109 bp. The primers of 18S: forward 5’-CACGGACAGGATTGACAGATT-3’, reverse 5’-GCCAGAGTCTCGTTCGTTATC-3’, product 119 bp. The protocol for real-time RT-qPCR were: a single cycle of reverse transcription at 50°C for 30 minutes, a single initial denaturation (95°C, 15 minutes), 40 cycles of denaturation (94°C, 15 seconds), annealing (57°C, 15 seconds) and extension (72°C, 30 seconds) and a single cycle of final extension (72°C, three minutes), which was continued with melt curve (at a range temperature of 72°–95°C and increasing 0.5°C for each cycle, wait for two seconds, and do the Aquire to melt A on green one time. Real-time RT-qPCR data was calculated according to Livak method.
Statistical analysis
The levels of HIF-1α were compared with unpaired t test. Oxygen venous saturation, level of MDA, GSH, IVH, and relative ratio mRNA were compared by Mann Whitney test. Protein expression and association between protein expression with intra ventricular hemorrhage were analyzed by using Chi-square test.
RESULTS
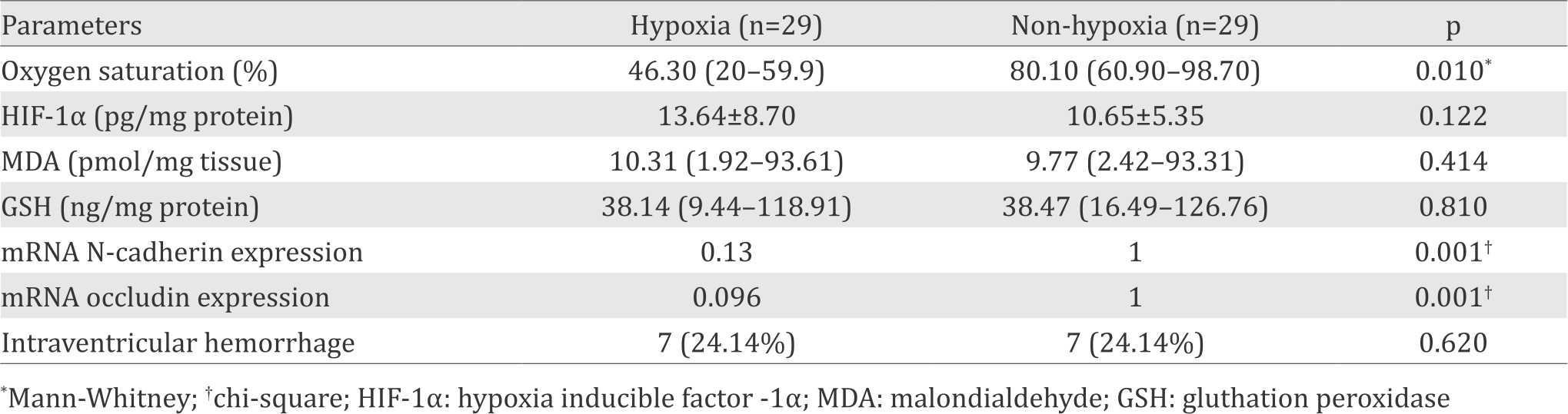
Twenty nine placentas of preterm infants in both groups have been colected and used for measurement of study parameters. Oxygen saturation of umbilical vein in hypoxic group {46.30% (range 20.0 to 59.9%)} was significantly lower than that of non-hypoxia group {80.10% (range 60.9% to 98.7%)}, p=0.001). The mean value of HIF-1α protein level in placenta of hypoxic group was slightly, but not significantly higher than in non-hypoxia group (p=0.122). The same phenomenon was also observed for MDA (p=0.414) and GSH (p=0.810) levels. By using the Livak method, we found that the relative mRNA expression of N-cadherin in hypoxia group was significantly weaker than those in the non-hypoxia group (0.13 vs 1, p=0.001); and the relative mRNA expression of occludin in hypoxia group was also significantly weaker compared to non-hypoxia group (0.096 vs 1, p=0.001). (See Table 1).
Table 1. Comparison between hypoxia parameter indicators in the placental tissue of hypoxic and non-hypoxic preterm infants
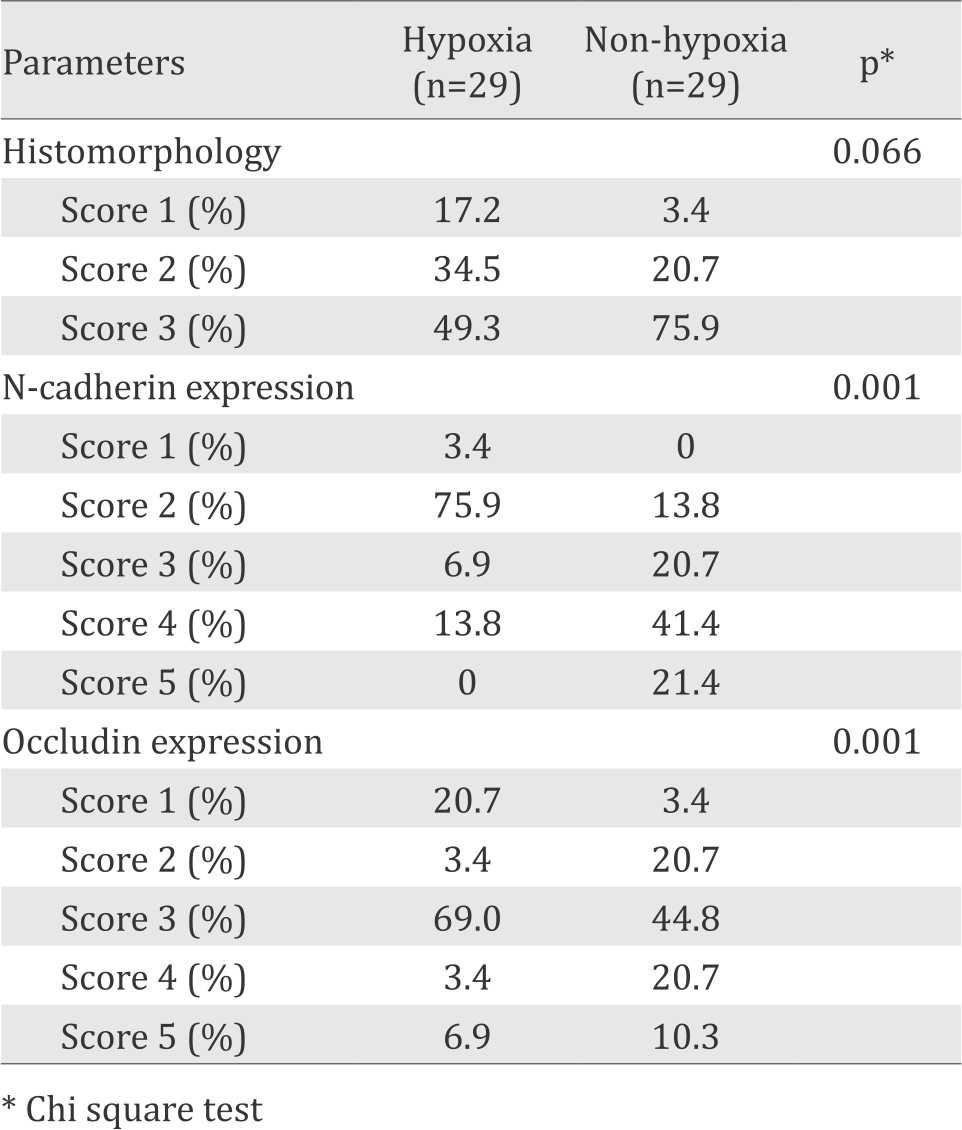
Using modification score of Moenadjat24 we found that the endothelial layer of placental capillary in hypoxia group was more intacts than in non-hypoxia group, although the difference was not statistically significant (p=0.066). Immunohistochemistry of N-cadherin expression in hypoxia group was significantly weaker than those in non hypoxia group (p=0.001), as well as occludin (p=0.001) (Table 2).
Table 2. Comparison between histomorphology an immunohistochemistry parameters in the placental tissue of hypoxic and non-hypoxic preterm infants
Histomorphology of placental capillary endothelial demonstrated that intact basal membrane and endothelial cells weare tightly attached to the basal membrane in non-hypoxia group. In contrast, basal membrane was not intact and some of endothelial cells were not tightly attached to the basal membrane in hypoxia group (Figure 1).
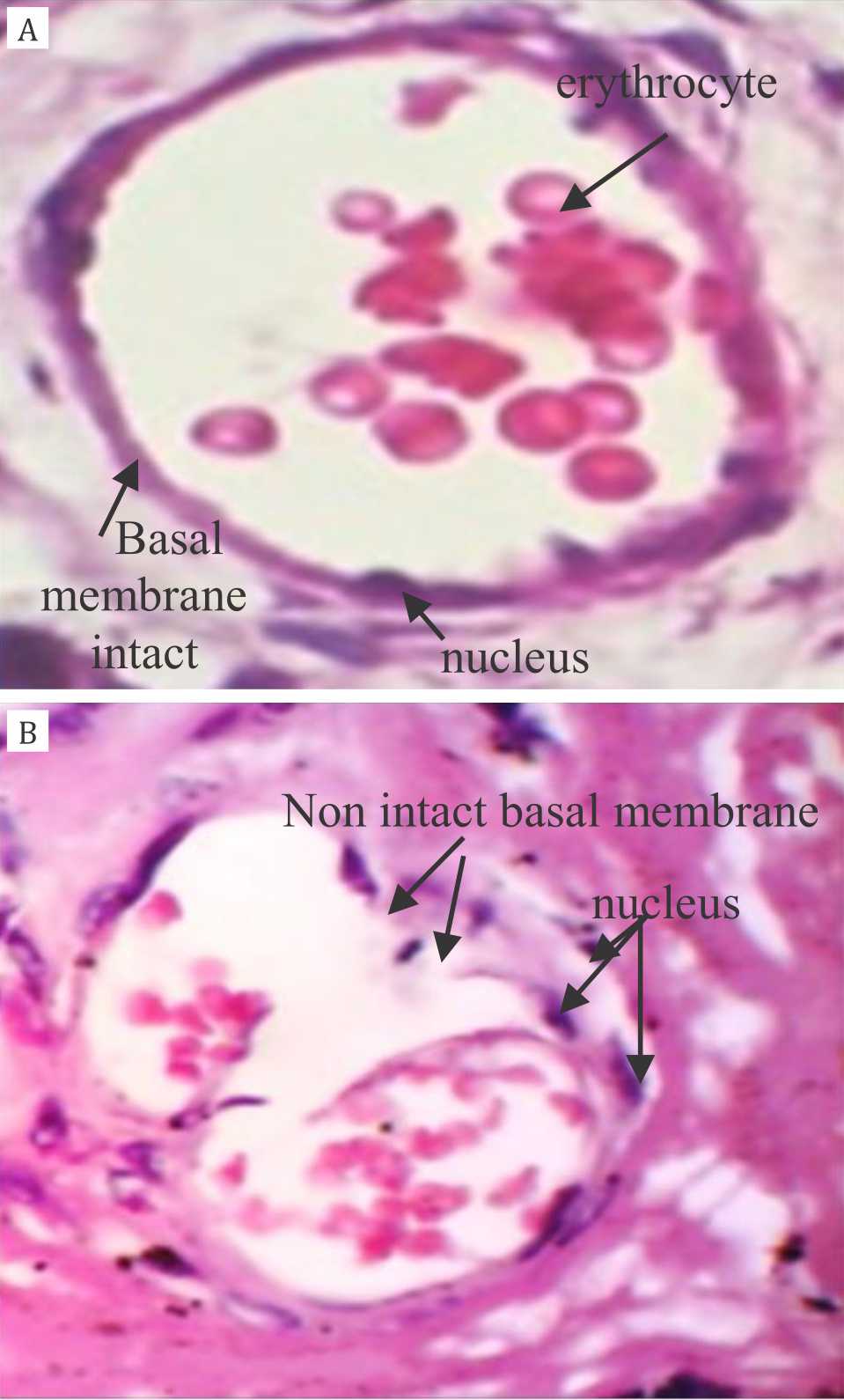
Figure 1. Histomorphology of placental capillary endothelial layer HE staining, 40x magnification. Panel A: intact basal membrane and endothelial cells are tightly attached to the basal membrane in non-hypoxia group; Panel B: basal membrane is not intact and some of endothelial cells are not tightly attached to the basal membrane in hypoxia group
The expression of N-cadherin protein on endothelial layer of placental capillary showed an intact basal membrane and endothelial cells attached tightly to basal membrane in nonhypoxic group; while on hypoxia group, an incomplete basal membrane could be detected with some of endothelial cells not tightly attached to basal membrane (Figure 2).
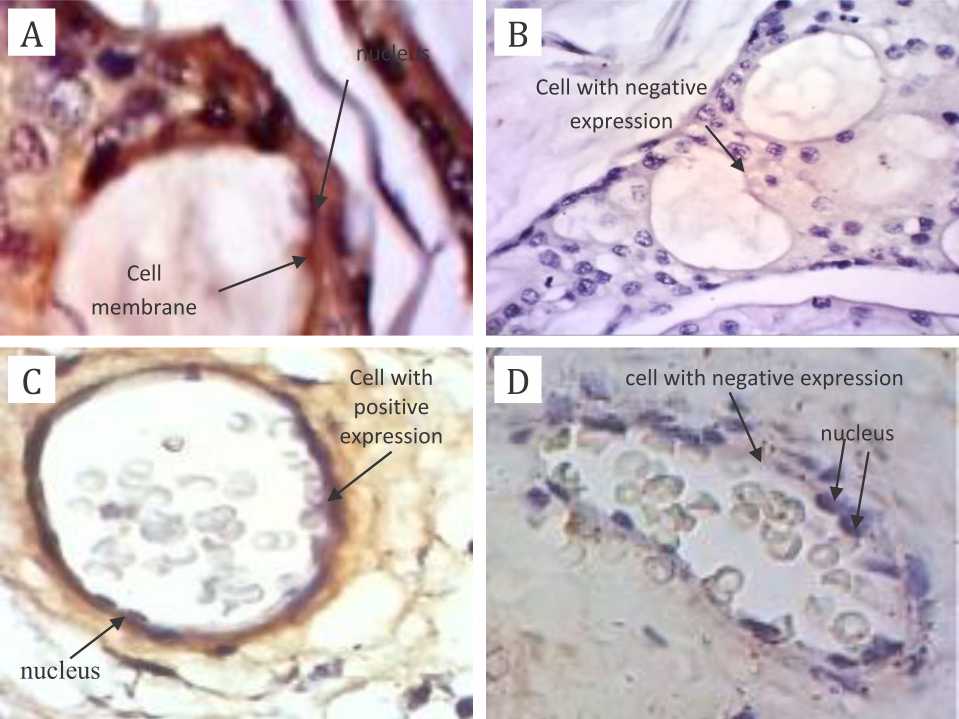
Figure 2. The expression of N-cadherin protein on endothelial layer of placental capillary, 40X magnification. A) positive control; B) negative control; C) non-hypoxia specimens; D) hypoxia specimens. Primary antibody: anti-N-cadherin antibody mouse monoclonal to N-cadherin; secondary antibody: novolink polymer. There are complete basal membrane and endothelial cells attached tightly to basal membrane in panel A and C; while on panel B and D, there are incomplete basal membrane and some of endothelial cells are not tightly attached to basal membrane
Figure 3 shows the expression of occludin protein on endothelial layer of placental capillary. The complete basal membrane with endothelial cells attached tightly to basal membrane was seen in non-hypoxic group, while a thin layer of basal membrane was seen in hypoxic group (Figure 3).
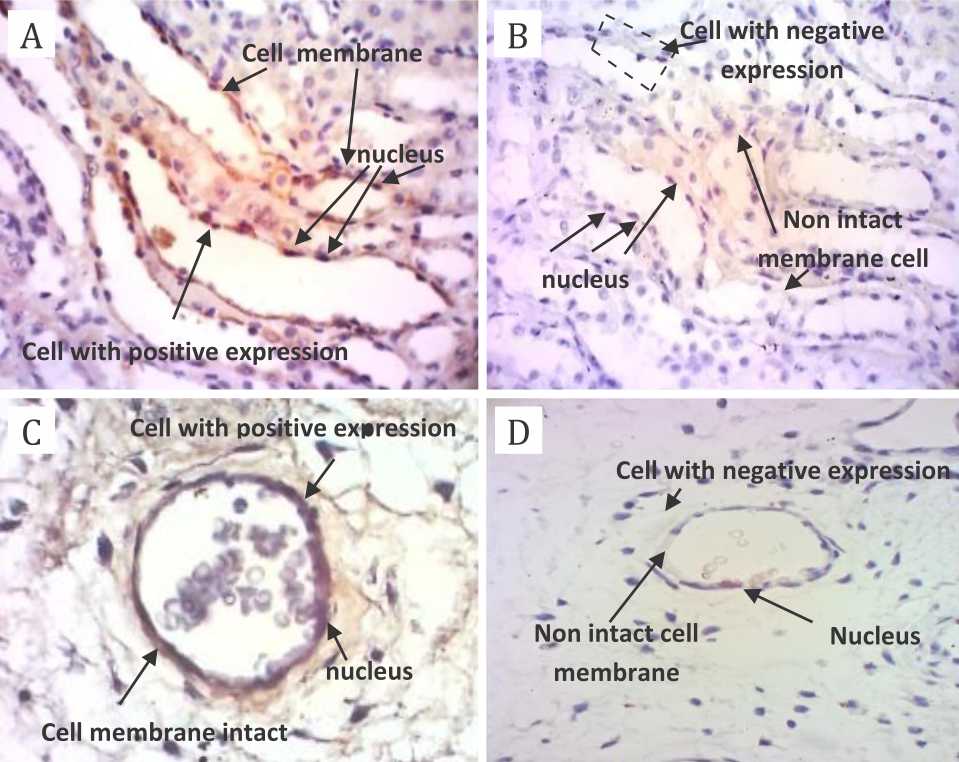
Figure 3. The expression of occludin protein on endothelial layer of placental capillary using light microscope with 40x magnification. Primary antibody: anti-occludin antibody rabbit polyclonal to occludin; secondary antibody: novolink polymer. A) positive control; B) negative control; C) nonhypoxia specimens; D) hypoxia specimens. There are complete basal membrane and endothelial cells attached tightly to basal membrane in panel A and C; while on the contrary, there is a thin layer of basal membrane in panel B and D
In the present study, intraventricular hemorrhage occurred in seven out of newborn in hypoxia and non-hypoxia group.
DISCUSSION
In the present study, we have tried to evaluate the role of HIF-1α and oxidative stress in the regulation of vascular integrity of umbilical vein using placental tissue of hypoxic and non-hypoxic preterm infants. This was done in an attempt to explain the phenomenon of intraventricular hemorrhage of preterm infants. mRNA relative expression and protein expression of N-cadherin and occludin, the proteins that maintain the integrity of capillary, was also evaluated. In addition, visualization of vascular integrity was also done by histomorphology and immunohistochemistry methods.
Response of placental tissue to hypoxia was indicated by the higher level of HIF-1α in hypoxia group compared to non-hypoxia group, although no significant difference was observed between the two groups. The mechanism of HIF-1α regulation by reactive oxygen species (ROS) in oxidative stress has not been fully understood. However, ROS probably induces HIF-1α accumulation by inhibiting its degradation, and even activating its transcription through adenosine monophosphate (AMP) kinasedependent mechanism. The regulation of HIF-1α by monophosphate-activated protein kinase (AMPK) as a response to ROS occurs through c-jun terminal kinase and Janus kinase pathways.25 The results of our study are consistent with the general characteristics of increased HIF-1α level in various tissues as have been shown by other studies.15,26–28 Increased mitochondrial ROS can induce various pathways of signal transduction that may lead to gene expression. ROS can activate tyrosine kinase receptor or inhibit phosphatase protein or activate mitogen activated protein kinase (MAPK) pathway, phospholipase CY (PLCϒ), protein kinase-C (PKC) and then activate transcription factors and target gene expression, including HIF-1α.27
Oxidative stress occurred in hypoxic placenta can be detected by measuring the level of MDA, which is a product of basal membrane lipid peroxidation process,10,11 and by measuring the endogenous antioxidant, GSH level.29,30 In our study, the MDA level was higher in hypoxia group compared to non-hypoxia group, and the GSH level was lower, indicating that oxidative stress has been occured in hypoxia condition, although the difference was not statistically significant.
Junction proteins of capillary endothelium can be directly impaired by free radicals produced by hypoxia or through signal transduction.31 Protein damage in the endothelial layer of placental capillary directly produces carbonyl compounds.8,31,32 In our study, impaired endothelial protein junction of placental capillary was indicated by the significantly weaker expression of endothelial junction protein in hypoxia group compared to non-hypoxia group. Lower expression of endothelial junction protein in placental capillary may cause the development of intercellular gap among endothelial cells, which increases the permeability of placental capillary endothelial layer.31 Oxidative stress due to hypoxia may cause reduced N-cadherin expression, either at protein or mRNA level. Theoretically, H2O2 initially activates tyrosine kinase and reduces the activity of cadherin/catenin phosphatase. Long-term exposure of H2O2 (24–48 hours or more) may inhibit phosphorylation p38 of MAPK families since the exposure of H2O2 may cause downstream of N-cadherin expression.33,34 Oxidative stress due to hypoxia may also explain lower expression of occludin junction protein, both at protein and mRNA level. Activation of myosin light chain kinase (MLCK) and it subsequent phosphorylation product, the myosin light chain (MLC), might have the role in the lower expression of endothelial occludin junctional protein, which may lead to lower occludin expression,35 as as well as lower relative occludin mRNA expression as demonstrated by the significantly lower expression of occludin protein expression and its relative mRNA in hypoxia group compared to non-hypoxia group of the present study.
The trend of lower protein expression and occludin relative mRNA expression might be related to reduced adenosine triphosphate (ATP) production during hypoxia, which subsequently inhibited phosphorylation, leading to further downstream of occludin expression, both at protein and mRNA level. Phosphokinase C, which is activated during hypoxia, can also reduce occludin and threonine phosphorylation, which lead to less tight junction between occludin and actin, and ultimately results in changes of molecular conformation.36
However, histomorphology analysis with hematoxylin-eosin (HE) staining, did not show any significant difference of endothelial integrity of placental capillary between the hypoxia and non-hypoxia group. Hypoxia may induce capillary fragility or interruption of cerebral blood flow which can destroy capillary endothelial junctional protein,17 and the increase risk of IVH can be expected. However, this study revealed that the incidence of intraventricular hemorrhage was not different between hypoxia and non-hypoxia, although the expresssion of junctional proteins, N-cadherin, and occludin, were significantly lower in hypoxia group. Explanation of this discrepancy was not fully understood. But, some mechanisms could be proposed. First, it is possible that the decrease of junctional protein expression was not sufficiently low to disrupt endothelial integrity. Secondly, the structural difference between the placental barrier and blood brain barrier might have resulted in different resistance. Blood brain barrier is rich of perivascular astrocytes foot process rendering smaller gap between its endothelial cells.3,5,7,17 In addition, other factors than hypoxia may have contributes in the occurence of IVH.17
In conclusion, placental hypoxia in premature infants in our study was associated with lower expression of endothelial protein junction of capillary placenta and may cause changes of placental capillary permeability. The changes of capillary placental protein expression did not correlate with intraventricular hemorrhage.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
This study was funded by research grant from Directorate of Research and Community Service, Universitas Indonesia, No. DRPM/RII/192/RMUI/ 2013.
REFERENCES
- Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition and fetal programming: role of the placenta. In: Kay HH, Nelson DM, Wang Y, editors. The Placenta. Oxford, UK:Wiley-Blackwell; 2011. p. 1–15.
- Frank HG. Placenta and Intrauterine Environment. In : Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 4th ed. Philadelphia. Elsevier Saunders. 2011; p. 108–20.
- Stolp H, Neuhaus A, Sundramoorthi R, Molnár Z. The long and the short of it: gene and environment interactions during early cortical development and consequences for long-term neurological disease. Front Psychiatry. 2012;3(50):1–22.
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901.
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
- González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778(3):729–56.
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16(2):209–21.
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. New York: Oxf Univ Press; 2007. p.30–337.
- Escobar J, Cernada M, Vento M. Oxygen and oxydative stress in the neonatal period. NeoReviews. 2011;12(11):613–24.
- Lata H, Ahuja GK, Narang APS, Walia L. Effect of immobilisation stress on lipid peroxidation and lipid profile in rabbits. Indian J Clin Biochem. 2004;19(2):1–4.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:1–31.
- Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292(5):H2023–31.
- Wang Y, Lewis DF, Alexander JS, Granger DN. Endothelial barrier function in preeclampsia. Front Biosci. 2007;12:2412–24.
- Trollmann R, Strasser K, Keller S, Antoniou X, Grenacher B, Ogunshola OO, et al. Placental HIFs as markers of cerebral hypoxic distress in fetal mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1973–81.
- Florio P, Perrone S, Luisi S, Vezzosi P, Longini M, Marzocchi B, et al. Increased plasma concentrations of activin a predict intraventricular hemorrhage in preterm newborns. Clin Chem. 2006;52(8):1516–21.
- Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders Elsevier; 2008. p. 517–86.
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8.
- Whitelaw A. Core concepts : intraventricular hemorrhage. NeoReviews. 2011;12(2):94–101.
- Bassan H. Intracranial hemorrhage in the preterm infant: understanding it, preventing it. Clin Perinatol. 2009;36(4):737–62.
- Morakami P. Pittfalls in interpreting umbilical cord blood gases and lactate at birth [dissertation]. Norway. Oslo University; 2013.p.15–30.
- Wills ED. Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B, editors. Biochemical toxicology: a practical approach. Irlandia Oxford University Press; 1987. p.127–52.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
- Jusuf HA. Panduan praktis histoteknik dasar dan pulasan jaringan rutin. Bagian Histologi Universitas Indonesia. 2011. p.1–34. Indonesian.
- Moenadjat Y. Disfungsi endotel dan penguraian endothelial junction pada luka bakar kritis dan luka bakar non kritis [dissertation]. Jakarta: Universitas Indonesia; 2012. p. 68–83. Indonesian.
- Jung SN, Yang WK, Kim J, Kim HS, Kim EJ, Yun H, et al. Reactive oxygen species stabilize hypoxia-inducible factor-1 alpha protein and stimulate transcriptional activity via AMP-activated protein kinase in DU145 human prostate cancer cells. Carcinogenesis. 2008;29(4):713–21.
- Wanandi SI, Dewi S, Paramita R. Ekspresi relatif mRNA HIF-1α pada jantung, otak dan darah tikus selama hipoksia sitemik. Makara Sains. 2009;13(2):185–8. Indonesian.
- Jusman SW. Respon jaringan hati terhadap hipoksia sistemik kronik: regulasi ekspresi gen sitoglobin oleh hypoxia-inducible factor-1α [dissertation]. Jakarta. Universitas Indonesia; 2010. p. 38–107. Indonesian.
- Conde E, Alegre L, Blanco-Sánchez I, Sáenz-Morales D, Aguado-Fraile E, Ponte B, et al. Hypoxia inducible factor 1-alpha (HIF-1 alpha) is induced during reperfusion after renal ischemia and is critical for proximal tubule cell survival. PloS One. 2012;7(3):e33258.
- Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15(4):191–5.
- Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23(2):161–6.
- Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280(4):C719–41.
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38.
- Kevil CG, Ohno N, Gute DC, Okayama N, Robinson SA, Chaney E, Alexander JS. Role of cadherin internalization in hidrogen peroxidase-mediated endothelial permeability. Free Radic Biol Med. 1998;24(6):1015–22.
- Hirata J, Ko JA, Mochizuki H, Funaishi K, Yamane K, Sonoda KH, et al. Oxidative stress regulates expression of claudin-1 in human RPE cells. Cent Eur J Biol. 2014;9(5):461–8.
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78(6):1223–32.
- Wang YL, Hui JN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia involving angiopoietin-1 signal way. Eye (Lond). 2007;21(12):1501–10.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id