
Section Abstract Introduction Case Illustration Discussion Conflict of Interest Acknowledgment References
Case Report
Dissolution of large intracardiac thrombus, potential role of the emerging oral fibrinolytic agent
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i1.1311 Med J Indones. 2016;25:62–6
Received: November 22, 2015
Accepted: February 03, 2016
Author affiliation:
Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia, National Cardiovascular Center Harapan Kita, Jakarta, Indonesia
Corresponding author:
Rony M. Candrasatria
E-mail: rony.candrasatria@ymail.com
Intracardiac thrombus may persist in some cases even after anticoagulant therapy. This opens a possibility to add a potent thrombolytic agent into therapeutic regimen without increasing bleeding risk any further. Increasing evidence showed a promising efficacy and safety of oral fibrin specific lumbrokinase as a thrombolytic agent. To the best of our knowledge, report of the use of lumbrokinase on intracardiac thrombus is limited. We reported two cases of intracardiac thrombi. In first patient, after two-month therapy with lumbrokinase, the previous 8 cm2 left atrial thrombus was completely disappeared. Second patient had left ventricular thrombus due to low left ventricular ejection fraction caused by coronary artery disease. A significant dissolution in thrombus size on repeated follow-up was found. Both patients did not experience any significant adverse effect. This case series aims to present the potential use of lumbrokinase as as oral antithrombotic therapy in intracardiac thrombus.
Keywords
intracardiac thrombus, lumbrokinase, oral fibrinolytic agent
Intracardiac thrombus, for example left atrial and left ventricular thrombus, is associated with several conditions especially low flow states such as in mitral stenosis, atrial fibrillation, and ischemic heart disease.1–4 Increased morbidity and mortality are associated with its embolization.3–6 The best antithrombotic strategy is not known and several studies showed that in some patients despite receiving anticoagulation, the thrombus may persist.7–10 This opens possibility to introduce a new therapeutic agent for significant dissolution without adding an increased risk of adverse effects especially bleeding.
Lumbrokinase is a group of enzymes possessing a strong thrombolytic activity extracted from earthworm (Lumbricus rubellus).11,12 Its clinical application is mostly confined in Asia. Several studies showed its efficacy in the treatment of coronary artery disease, stroke, and thromboembolic diseases.13–17 Despite its potent thrombolytic effect, lumbrokinase has a minimum bleeding risk due to its fibrin specific characteristic. This holds potential use as ideal thrombolytic adjuctive therapy or even as cornerstone in thromboembolism.18 Data about its use on intracardiac thrombus are limited. This case series aims to present the potential use of lumbrokinase as an ideal thrombolytic in intracardiac thrombus management.
CASE ILLUSTRATION
First case, a 32 years old male was referred to National Cardiovascular Center Harapan Kita, Jakarta, with severe mitral stenosis due to rheumatic heart disease, left atrial thrombus, atrial fibrillation with normal ventricular response, and history of ischemic stroke in 2001. Patient complained of shortness of breath since two weeks before outpatient appointment. Previously patient had history of shortness of breath upon activity for years. Patient received warfarin 2 mg once daily, ramipril 5 mg once daily, digoxin 0.25 mg once daily, furosemide 40 mg once daily, and hepatoprotector from local hospital. Warfarin was consumed irregularly without international normalized ratio (INR) check. Patient experienced stroke in 2001 without any current neurological sequelae.
Upon physical examination, patient showed normal blood pressure, breathing rate and temperature and irregular heart rate. Heart auscultation found mid diastolic murmur at apex and early diastolic murmur at left sternal border. Lung was clear upon auscultation. Electrocardiogram revealed an atrial fibrillation with normal ventricular rate. Blood test was within normal range except for serum glutamic oxaloacetic transaminase (SGOT) 246 U/L and serum glutamic pyruvic transaminase (SGPT) 455 U/L. Echocardiogram from referring hospital found a left atrial thrombus of approximately 8 cm2 with severe mitral stenosis and mildmoderate aortic regurgitation (Figure 1).
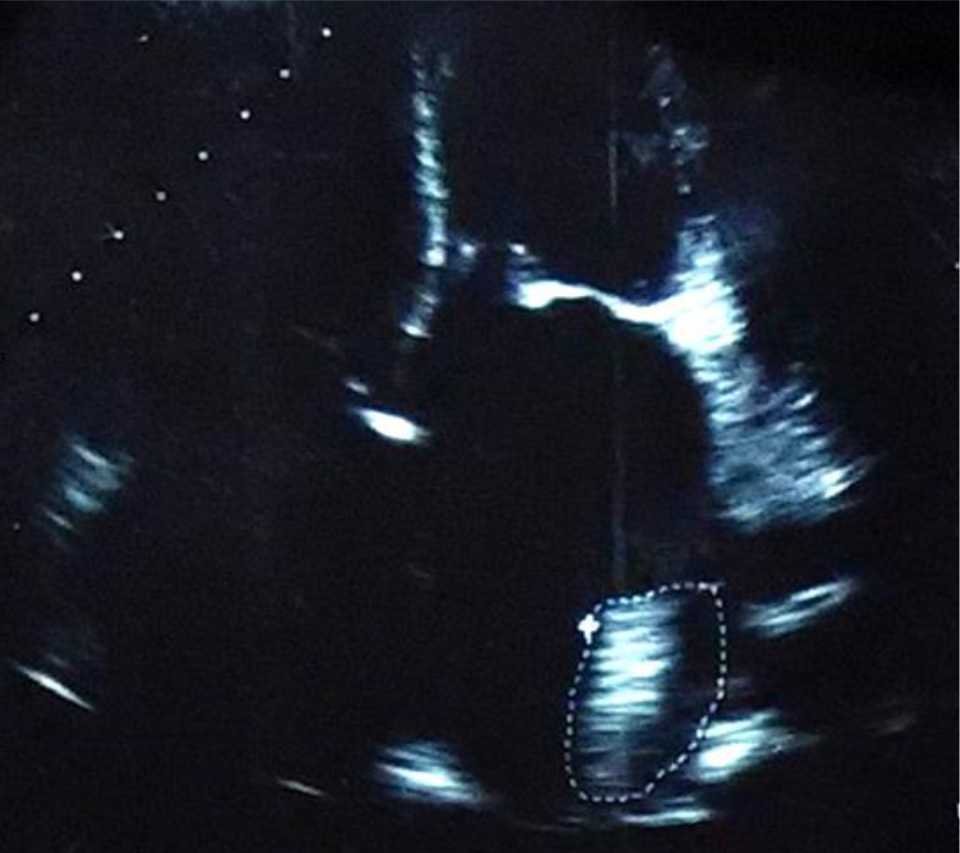
Figure 1. Four apical chamber view echocardiogram revealed a left atrial thrombus with approximate size of 8 cm2 (white dashed line)
We decided to continue warfarin 2 mg once daily and then adjusted to 2 mg once daily and every third day 4 mg once daily after INR check (INR 1.28). Lumbrokinase was added into therapy with doses of two capsules three times daily (each containing 250 mg of Lumbrokinase extract, equivalent to 300,000 unit Lumbrokinase). After one month of treatment, follow-up transthoracic echocardiogram showed a reduced thrombus into approximately 3.8 cm2 (figure 2). Lumbrokinase was then reduced to a capsule twice daily. On the following month, the thrombus was no longer found in follow-up echochardiogram with Wilkin Score 8, mitral valve area of 0.6 cm2, no mitral regurgitation, severe pulmonary hypertension, left ventricular ejection fraction (LVEF) 56%, and tricuspid annular plane systolic excursion (TAPSE) 1.1 cm (figure 3). Clinically, there were no significant adverse effects observed during the treatment course.
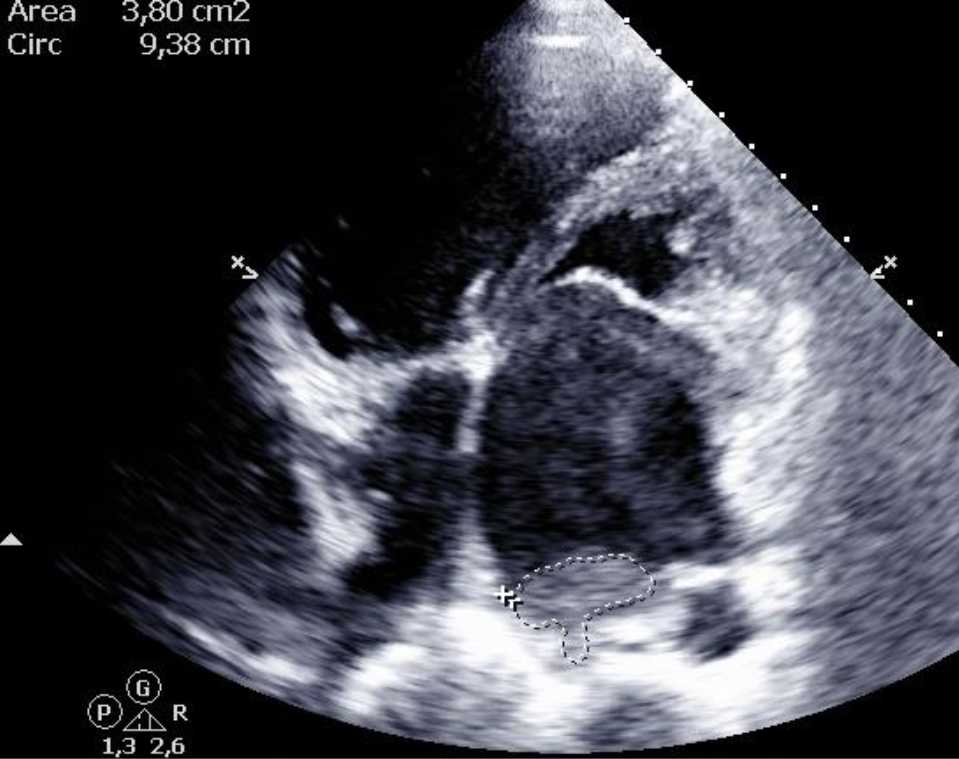
Figure 2. After a month of treatment with lumbrokinase, the size of thrombus decreased to 3.8 cm2 (White dashed line)
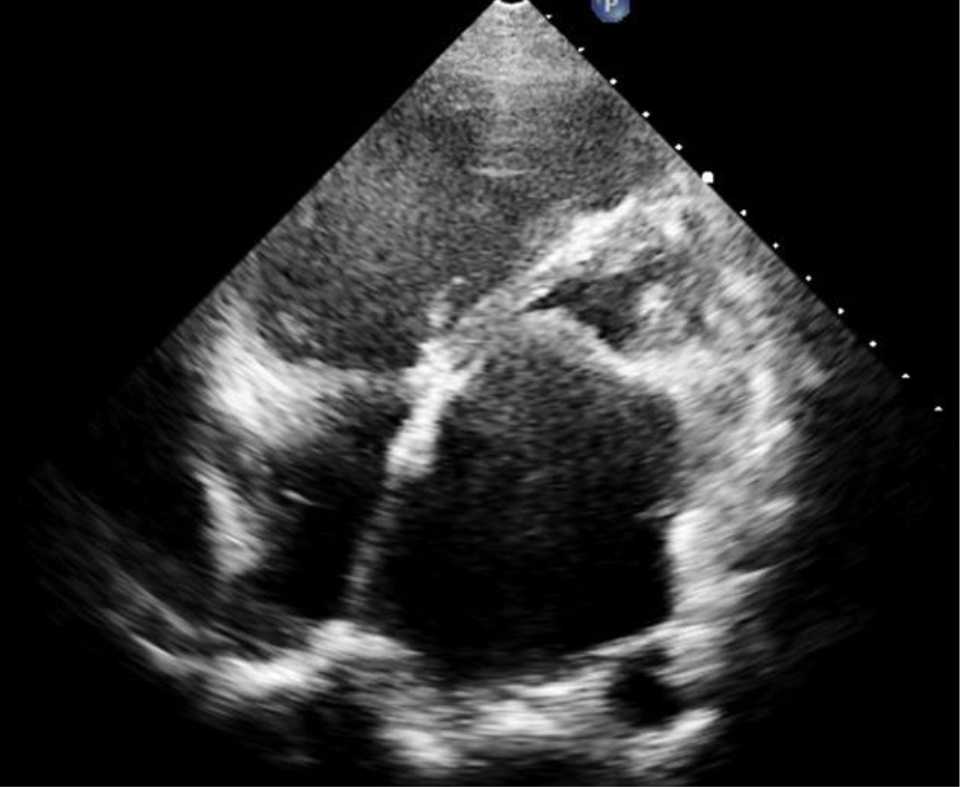
Figure 3. After the second month of treatment with lumbrokinase, the trombus was no longer found in transthoracic echocardiogram
Second case, a 51 years old male was referred from West Kalimantan with diagnosis of cardiomyopathy. He complained of frequent chest pain since one and a half year ago with the worst pain was felt three months ago. He received furosemide, spironolactone, trimetazidine, aspirin, and rosuvastatin from previous hospital. In outpatient clinic, physical examination was unremarkable. The electrocardiogram (ECG) revealed pathologic Q in lead V1-4 suggesting old anterior infarction. Chest X-ray showed enlarged heart with cardiothoracic ratio of 60%. Previous echocardiogram showed LVEF of 20%. Cardiac magnetic resonance imaging (MRI) was done then in our hospital with result of depressed LVEF (18%), depressed right ventricle systolic function, severe hypokinetic at septum, anterior, inferolateral and akinetic at apex; moderate hypoperfusion at septum and anterior during rest and stress perfusion, total scar volume of 29%, with thrombus seen at apex with size of 15.6 mm x 8.5 mm (figure 4). Coronary Computed Tomography (CT) scan revealed extensive calcium plaque burden with severe stenosis in left anterior descending artery (90%), left circumflex artery (90%) and total occlusion at distal right coronary artery. Lumbrokinase two capsules three times daily, clopidogrel, carvedilol, and ramipril were added into therapy. Three months later, MRI was repeated with result of improvement in LVEF (increased up to 27%), normal right ventricle systolic function (from previous 37% to 62%), stress inducible ischemia at left anterior descending artery territory, reduced scar volume to 20% and the thrombus reduced to 3 mm x 4 mm. Lumbrokinase was then continued with dosage of one capsule twice daily. Another MRI was repeated five months after with LVEF of 27%, normal right ventricle function, inducible ischemia in left anterior descending artery territory, and reduced thrombus to 1.9 x 0.4 mm (Figure 5).
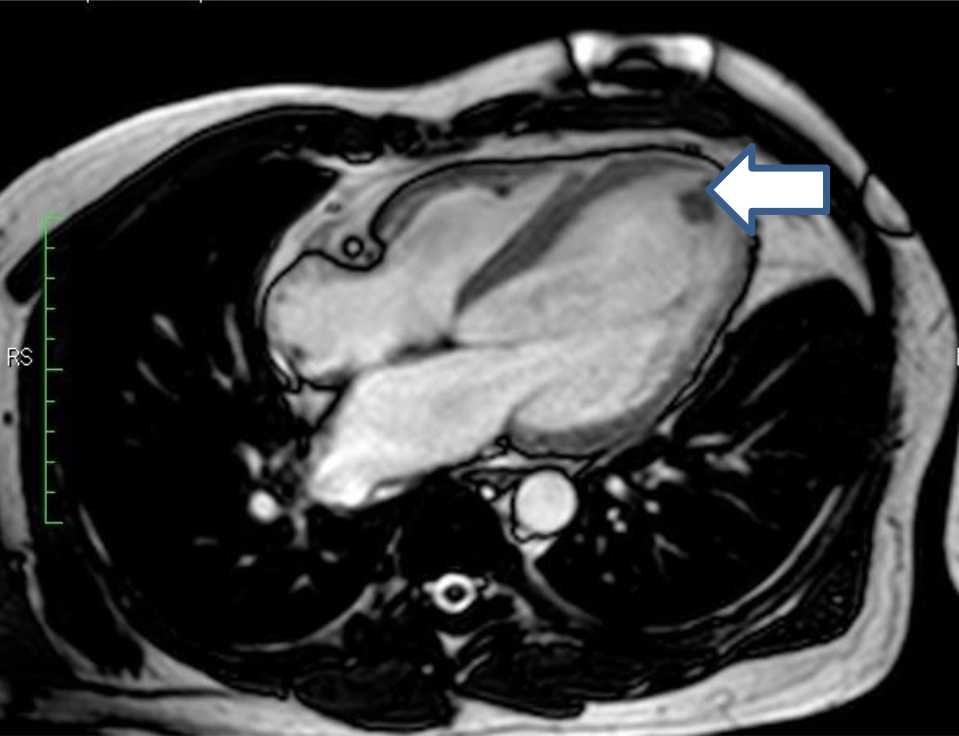
Figure 4. First magnetic resonance imaging (MRI) showed 15.6 mm x 8.5 mm thrombus at left ventricle apex (white arrow)
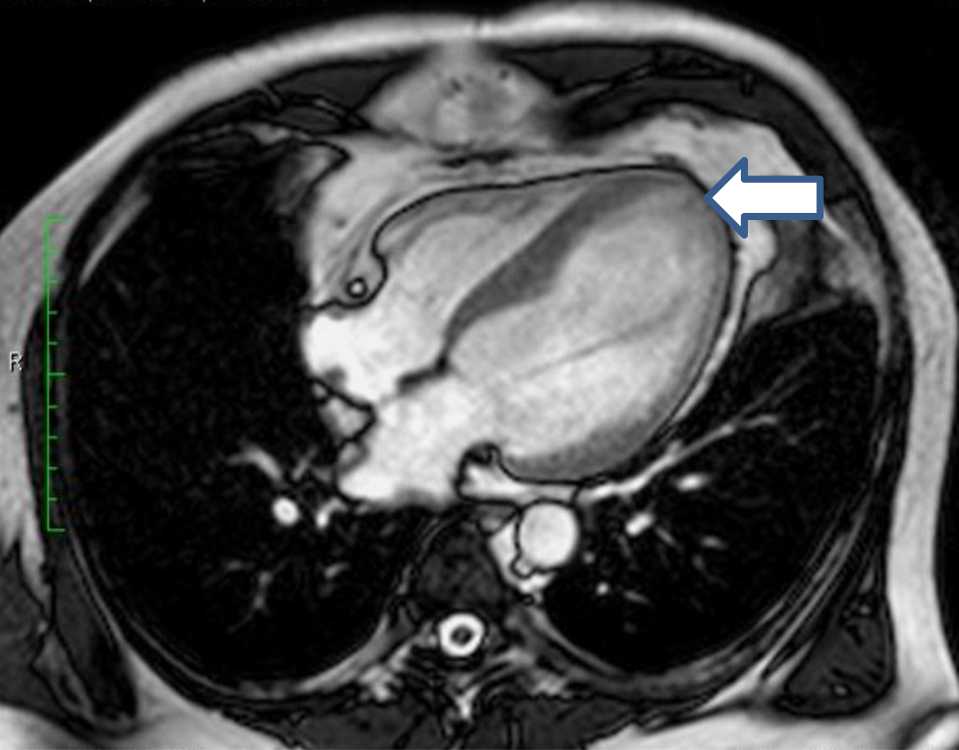
Figure 5. After eight months therapy with lumbrokinase, the thrombus was barely noticed, reduced to 1.9 x 0.4 mm (white arrow)
DISCUSSION
Left atrial thrombus is present in up to one third of patients with mitral stenosis and atrial fibrillation due to low flow states.7,19 Treatment is mandated to reduce the risk of thromboembolism especially in form of stroke. Complete dissolution of left atrial thrombus may not be fully achieved by traditional anticoagulants. Among candidates for percutaneous transvenous mitral commisurotomy who were given warfarin with INR maintained between 2.0 and 3.0 and followed up for 6 to 34 months, the disappearance rate of left atrial thrombus is only 62%.20 Cheol et al21 found six of nine patients with adequate anticoagulation with warfarin had persistent trombi eventhough the size of thrombi decreased from 2.2±0.8 cm to 0.9±1.0 cm.21 A study conducted by Fukuda et al8 revealed a better result, left atrial thrombus was found in 8.8% of patients with sub-therapeutic anticoagulation therapy and in 3.6% of patients with sufficient anticoagulation therapy. Nevertheless, this study was conducted in non valvular atrial fibrillation population which did not reflect our patient situation closely.8 Jaber et al9 found thrombus resolution in 80.1% of patients who received anticoagulation for 47±18 days. Contrast to plenty of data available on the usage of warfarin, the data of lumbrokinase for left atrial thrombus are limited. A study compared the usage of warfarin vs lumbrokinase in left atrial thrombus for 45 days revealed a comparable efficacy. Disappearance rate is 83.8% and 82.3% for lumbrokinase and warfarin respectively.22 Those available data suggest that single antithrombotic therapy may not be sufficient for treating left atrial thrombus. Using nomogram developed by Silaruks et al20 for predicting the dissapearance of left atrial thrombus after warfarin therapy, our patient has approximately 25% chance of left atrial thrombus disappearance, mostly due to its large size. The less likelihood of complete dissolution opens the possibility to use lumbrokinase as adjunctive therapy to increase the likelihood for disappearance. Lumbrokinase was chosen due to its potent fibrinolytic activity and low bleeding risk.18,22 Due to its fibrin specific profile, addition to warfarin therapy is expected not to increase bleeding risk any further.
Our first patient had CHA2DS2-VASc score of three (the score consists of congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, and sex category) which corresponds to high risk of stroke who required oral anticoagulant of warfarin or novel anticoagulants as per European Society of Cardiology guidelines.23 Indeed, patient had history of stroke four years ago. Warfarin had been consumed previously with insufficient INR level due to incompliance. In a small study, low intensity warfarin therapy resolved 71% left atrial thrombi in non valvular atrial fibrillation. Thus a combined effect of warfarin and lumbrokinase may explain the complete dissolution of large atrial thrombus in our patient despite its low chance of disappearance (25%, using nomogram developed by Silaruks et al20). Upon several followups, no significant adverse effect including bleeding was observed in our patient.
In the second case, patient had left ventricular thrombus due to low LVEF caused by coronary artery disease. Patient received clopidogrel and lumbrokinase without warfarin. Meurin et al10 in a prospective multicenter study found most thrombi (92.3%) disappeared after triple antithrombotic therapy (warfarin in addition to dual antiplatelet therapy). The thrombus in this study was a new thrombus which is more likely to respond to therapy. In our case, the thrombus was more likely to be old thrombus which was persisted after antiplatelet therapy. In addition to improvement in left and right ventricular systolic function and reduced scar volume, the trombus was significantly reduced after treatment with lumbrokinase without any significant adverse effect. These finding may imply potential role of lumbrokinase in improving ischemic profile and dissolute intracardiac thrombus.
In conclusion, lumbrokinase may play a role as a antithrombotic therapy in large intracardiac thrombus. Further clinical trial is needed to validate its efficacy and safety.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
Thromboles was used as source of lumbrokinase in this case supported by Biolife medilab.
REFERENCES
- Karthikeyan G, Thachil A, Sharma S, Kalaivani M, Ramakrishnan L. Elevated high sensitivity CRP levels in patients with mitral stenosis and left atrial thrombus. Int J Cardiol. 2007;122(3):252–4.
- Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol. 1995;25(6):1354–61.
- Zotz RJ, Pinnau U, Genth S, Erbel R, Meyer J. Left atrial thrombi despite anticoagulant and antiplatelet therapy. Clin Cardiol. 1994;17(7):375–82.
- Lip GY. Intracardiac thrombus formation in cardiac impairment: the role of anticoagulant therapy. Postgrad Med J. 1996;72(854):731–8.
- Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28(10):973–7.
- Malik R, Alyeshmerni DA, Wang Z , Goldstein SA, Torguson R, Lindsay J, et al. Prevalence and predictors of left atrial thrombus in patients with atrial fibrillation: is transesophageal echocardiography necessary before cardioversion? Cardiovasc Revasc Med. 2015;16(1):12–4.
- Srimannarayana J, Varma RS, Satheesh S, Anilkumar R, Balachander J. Prevalence of left atrial thrombus in rheumatic mitral stenosis with atrial fibrillation and its response to anticoagulation: a transesophageal echocardiographic study. Indian Heart J. 2003;55(4):358–61.
- Fukuda S, Watanabe H, Shimada K, Aikawa M, Kono Y, Jissho S, et al. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. J Cardiol. 2011;58(3):266–77.
- Jaber WA, Prior DL, Thamilarasan M, Grimm RA, Thomas JD, Klein AL, et al. Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. Am Heart J. 2000;140(1):150–6.
- Meurin P, Brandao Carreira V, Dumaine R, Shqueir A, Milleron O, Safar B, et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low ventricular ejection fraction: a prospective multicenterstudy. Am Heart J. 2015;170(2):256–62.
- Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikeda R, Seiki M, et al. A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn J Physiol. 1991;41(3):461–72.
- Pan R, Zhang ZJ, He RQ. Earthworm protease. Appl Environ Soil Sci. 2010;2010:1–13.
- Jin L, Jin H, Zhang G, Xu G. Changes in coagulation and tissue plasminogen activator after the treatment of cerebral infarction with lumbrokinase. Clin Hemorheol Microcirc. 2000;23(2-4):213–8.
- Pang SQ, Ding MC, Xie SP, Chai XQ, Deng LY, et al. A clinical study of therapeutic effectiveness in treating ischemic cerebrovascular disease with lumbrokinase. Chin J Neurol Psychiatry. 1993;26(4):229–31.
- Kasim M, Kiat AA, Rohman MS, Hanifah Y, Kiat H. Improved myocardial perfusion in stable angina pectoris by oral lumbrokinase: a pilot study. J Altern Complement Med. 2009;15(5):539–44.
- Ahmed K, Munawar M, Munawar DA, Hartono B. Impact of oral thrombolysis after catheter-based thrombectomy in acute and subacute submassive pulmonary thromboembolism. Chin Med J. 2015;128(3):401–3.
- Cao YJ, Zhang X, Wang WH, Zhai WQ, Qian JF, Wang JS, et al. Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: results from a multicenter, randomized, parallelgroup and controlled clinical trial. Chin Med J. 2013;126(21):4060–5.
- Verma MK, Pulicherla KK. Lumbrokinase – a potent and stable fibrin–specific plasminogen activator. Int J Bio-Sci Bio-Tech. 2011;3(2):57–70.
- Angjushev MK, Lazarevska M. Left atrial and left atrial appendage mass diagnosed by cardiac imaging: a case report. Arch Cardiovasc Imaging. 2014;2(1):e15633.
- Silaruks S, Thinkhamrop B, Tantikosum W, Wongvipaporn C, Tatsanavivat P, Klungboonkrong V. A prognostic model for predicting the disappearance of left atrial thrombi among candidates for percutaneous transvenous mitral commissurotomy. J Am Coll Cardiol. 2002;39(5):886–91.
- Kim CH, Park SW, Zo JH, Oh BH, Lee MM, Seo JD, et al. Evolution of left atrial thrombus with anticoagulant therapy-follow-up by transesophageal echocardiography. Korean J Intern Med. 1995;10(2):143–5.
- Feng SQ, Ting ZA. Lumbrokinase versus warfarin in treating left atrial thrombosis. J Med Forum. 2006;27(13):110.
- Camm AJ, Lip GY, De Catarina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the Europan Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id