
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Association of ATP-binding cassette sub-family B member 1 gene C3435T polymorphism with neutropenia in breast cancer patients treated with chemotherapy
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i3.1326 Med J Indones. 2016;25:156–62
Received: December 10, 2015
Accepted: March 07, 2016
Author affiliation:
1 Biomedical Master Programme, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
2 Department of Pharmacology and Therapeutic, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
3 Department of Surgery, Oncology Subdivision, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
4 Department of Biochemistry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Corresponding author:
Siti Syarifah
E-mail: syarifah8512@yahoo.com
Background
Neutropenia is the most common adverse event of breast cancer chemotherapy which can be life threatening due to opportunistic infection, neutropenic episodes may lead to delay or reduction of drug doses which may compromise treatment outcomes. In this study, we investigated the association of ATP-binding cassette subfamily B member 1 (ABCB1) gene C3435T polymorphism with the grading of neutropenia in breast cancer patients who treated with doxorubicin-taxan.
Methods
72 Indonesian female breast cancer patients from Haji Adam Malik Hospital who had been diagnosed and treated with doxorubicin-taxane regimen were selected for this cohort study. DNA was extracted from peripheral leucocytes and ABCB1 C3435T polymorphism was analyzed with PCR-RFLP. Patient data were collected from patient’s medical record for 3 cycles of chemotherapy. Association between ABCB1 C3435T polymorphism with neutropenia was assessed using Kruskal-Wallis test. Decline of absolute neutrophil count was assessed using Wilcoxon test. Genotype deviation and allele frequencies were also determined by Hardy-Weinberg Equilibrium.
Results
The frequencies of ABCB1 C3435T genotype for wildtype (CC), heterozygous (CT) and homozygous mutant (TT) was 22 (30.6%), 38 (52.8%) and 12 (16.7%) respectively. No association were found between ABCB1 C3435T polymorphism and the grading of neutropenia (p>0.05). There was a difference on the average of absolute neutrophil count after the first chemotherapy and after the third chemotherapy (p<0.05). There was no significant deviation of allele and genotype frequency from Hardy- Weinberg Equilibrium.
Conclusion
ABCB1 C3435T polymorphism had no association with the grading of neutropenia in breast cancer patients treated with doxorubicin-taxane regimen, however there was a trend of absolute neutrophil count declining during the 3 cycles of chemotherapy.
Keywords
ABCB1 C3435T polymorphism, breast cancer, doxorubicin-taxane, neutropenia
Breast cancer is the most common cancer among women who live in developed and developing countries with 1.38 million women newly diagnosed worldwide.1 In Indonesia, breast cancer is the most common cancer among women with incidence rates of 26:100,000 women and breast cancer patients have the highest number of hospitalization with 16.86%. The medical record of Adam Malik Hospital located in Medan, North Sumatera showed 532 women were hospitalized due breast cancer in 2012 and majority of cases were diagnosed in the late stages.2
Chemotherapy is an important breast cancer treatment that not only alleviate survival rates, but also cause various adverse events. Neutropenia is the most common adverse event which can be life threatening due to opportunistic infection. Neutropenic episodes may lead to delay and/or reduction of drug doses which may compromise treatment outcomes.3 C3435T polymorphism in exon 26 of ATP-binding cassette sub-family B member 1 (ABCB1) which encode P-glycoprotein (P-gp) was considered to be associated with an increasing risk of neutropenia. P-gp is an energydependent multidrug efflux pump, known as multidrug transporter for many various toxins including carcinogenic agents and anticancer agents such as antracycline, taxane, alcaloid vinca, epidopolitoxin and tamoxifen. P-gp is normally expressed in the epithelial cells of kidney, liver, gastrointestinal track, adrenal gland, blood brain barrier and hematopoietic stem cells.4
ABCB1 gene, localized to chromosome 7q21, contains 28 exons, ranging in size from 49 to 587 bp. ABCB1 gene codes P-gp which consist of 1,280 amino acids. The ABCB1 gene is polymorphic and 50 single nucleotide polymorphisms (SNP) has been identified so far. The most important ABCB1 gene polymorphism is the C3435T. Polymorphism of ABCB1 C3435T changes the structure and function of P-gp. The C3435T polymorphism of ABCB1 gene is a change of one base in nucleotide position 3,435 in exon 26 from cytosine (C) to thymine (T). Eventhough this SNP is synonymous which does not result in change of amino acid. The change in isoleucin (Ile1145Ile) changes P-gp function. The C3435T is the most commonly studied since this SNP has a high frequency in African-American population (10%), and Caucasian and Asian Population (40%–50%).5
Doxorubicin is the first anthracycline which was produced from Streptomyces peucetius (var. caesius) in the early 1960s. Doxorubicin has a broad-spectrum activity. Doxorubicin acts by intercalating deoxy ribonucleic acid (DNA), inhibition of topoisomerase II, formation of reactive oxygen species and lipid peroxidation. Taxanes is an antimicrotubulus drug group which consists of paclitaxel and docetaxel. Antitumor activity of the taxane group is the result of the drug binding with beta subunit of tubulin which stabilizes tubulin polymerisation. Acute toxicity which can happen after the administration of doxorubicin-taxane is myelosupression in which neutropenia is more common to happen than trombocytopenia.6
A lot of attention is given to ABCB1 C3435T polymorphism, which is the most prevalent SNP, to see P-gp expression in different tissues of human being and in different ethnicity. Presentation of T allele in C3435T decreases P-gp level in Caucasian, Chinese, Philipinos, Portugese, and Saudi Arabians.4,5 Despite of ABCB1 C3435T resulting in synonymous isoleucine to isoleucine change, it has been observed that individual homozygous for the T-allele had a two-fold lower level of P-gp expression compared with individual homozygous for the C-allele.5
In vitro and in vivo studies showed that this silent polymorphism changes the folding and function of P-gp. The wobble codon is considered to be responsible for the changes in protein translation.7 Homozygous variant (TT) have declining absolute neutrophil count, about 1.5 times lower compared to the wildtype group (CC).8 This study is relevant with prior observation that T allele is associated with declining P-gp expression in liver and the declining of CD56+ cell activity, an effect associated with declining messenger ribonucleic acid (mRNA) stabilization as a result of this SNP.9
In this study, we investigated the association of ABCB1 gene C3435T polymorphism with the grading of neutropenia in breast cancer patients.
METHODS
Seventy two female Indonesian breast cancer patients from Haji Adam Malik Hospital, Medan, who had been diagnosed and treated with doxorubicin-paclitaxel regimen were selected for this cohort study from February to August 2014. The patients were interviewed using case report form in order to find out several patient’s data and underwent physical examination and laboratory test. Based on approved case report form list, selection of patients had been made which fulfilled inclusion and exclusion criteria. Physical examination has been done by physician in oncological polyclinic of Department of Surgery Haji Adam Malik Hospital, Medan. Physical examination data are body weight, height (for determine body surface area), Karnofsky score, stadium tumor-node-metastasis (TNM) also determined. The result of pathological anatomy examination was determined by pathological anatomy specialist. Laboratorium test has been done in Clinical Pathology Lab of Haji Adam Malik Hospital, medan. Laboratory test data are complete blood count (CBC) examination, kidney function test (ureum, creatinin), and liver function test serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT). Informed consent was obtained from all subjects. Eligible patients had histologically confirmed breast cancer, had been planned to receive paclitaxel-doxorubicin regimen, ages 16–68 years old, had normal liver function and kidney function, had a normal CBC. Patient who smoked, had a history of cardiac disease, hematologic disease, had infection, consumed drug induced neutropenia (antipsychotic, anticonvulsant) and had previous radiotherapy in the last three months were excluded from this study. This protocol of this study has been approved by Medical Ethics Committee University of North Sumatera (No. 27/KOMET/FK USU/2014).
Genomic DNA was isolated from peripheral leukocyte. The amplification of isolated DNA was performed with polymerase chain reaction (PCR) with each reaction containing DNA GoTaq® Green Master Mix (Promega), forward primer (5’-TGTTTTCAGCTGCTTGATGG-3’) and reverse primer (5’-AAGGCATGTATGTTGGCCTC-3’), nuclease free water, and DNA template with the final volume of 25 μl. The PCR cycle consisted of an initial two minutes denaturation at 94°C, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds and extension at 72°C for 30 seconds, the final elongation was performed at 72°C for seven minutes.
The ABCB1 C3435T polymorphism was analyzed with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Briefly, 5 μl of PCR product was digested by one unit restriction endonuclease enzyme Sau3AI (Promega) at 37°C for two hours. Digested products were separated by 2% agarose gel electrophoresis and observed directly under ultraviolet (UV) light after staining with ethidium bromide. Electrophoresis patterns showed 158 bp fragment for homozygous wildtype (CC), 197 and 158 bp fragment for heterozygous variant (CT), and 197 bp fragment for homozygous variant (TT). Neutropenic data and characteristic of subjects were collected from medical records for three cycles of chemotherapy. Neutropenia was classified into grade 1–4 based on Common Terminology and Criteria of Adverse Events v.4.0 (CTCAE).10
Statistical analysis was performed by applying statistical package for social sciences software (SPSS v.19.0). Kruskal-Wallis test was performed to check the association of polymorphism with grading of neutropenia. Analysis of declining absolute neutrophil count trend was checked by Wilcoxon test. P values less than 0.05 were considered significant. Distribution of allele frequency and genotype was calculated by Hardy-Weinberg Equilibrium.
RESULTS
Characteristics of subjects can be seen in Table 1. Frequencies of CC, CT and TT genotypes were 22 (30.6%), 38 (52.8%), and 12 (16.7%) respectively. The electrophoresis pattern of ABCB1 C3435T can be seen in Figure 1. We found four ethnics that were Bataknese, Minangkabau, Javanese, and Acehnese. Distribution of ABCB1 C3435T was varied among these ethnics. Bataknese had the highest frequency of homozygous CC genotype (wildtype) with 12 (54.50%), Javanese had the highest frequency of heterozygous variant (CT) with 21 (55.30%) and homozygous variant (TT) was the lowest among all ethnic groups. There was no association between ABCB1 C3435T polymorphism with the grading of neutropenia (p>0.05) for post-chemotherapy cycle 1, cycle 2 and cycle 3. We can see from Table 2.
Table 1. Characteristic of subjects

Table 2. Association of ABCB1 C3435T with neutrophil grading post-chemotherapy cycle I, cycle II, and cycle III
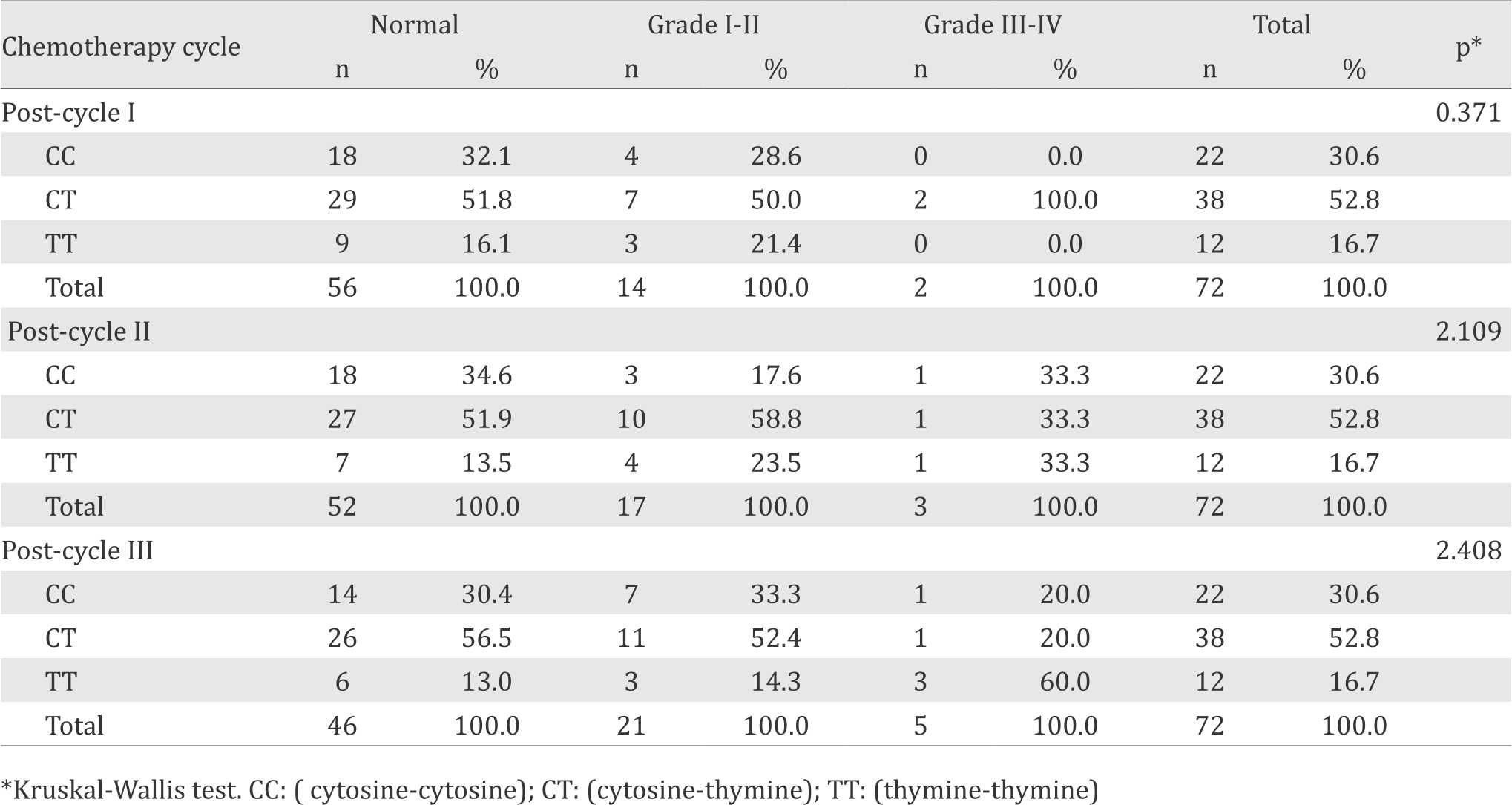
Figure 1. Electrophoresis pattern of ABCB1 C3435T by PCRRFLP: CC genotype (C, D), TT genotype (E), CT genotype (F,G,H), 100 bp DNA ladder marker (A), Undigested PCR product (B)
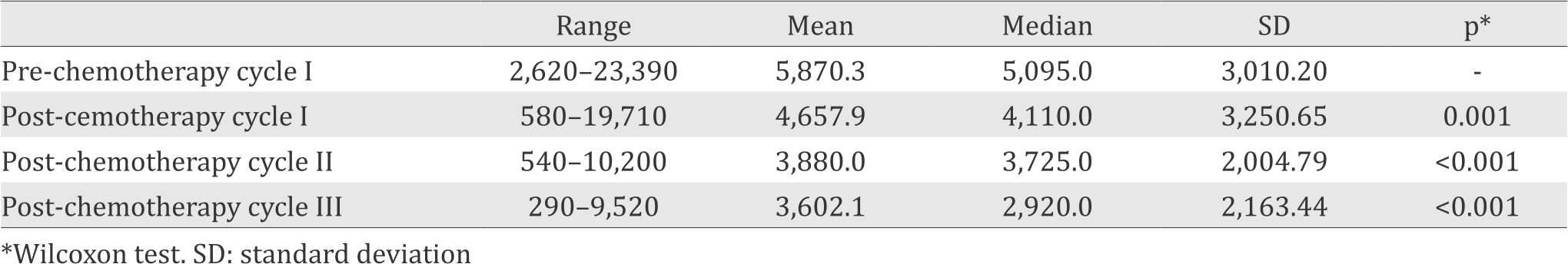
In this study, no association between polymorphism of ABCB1 gene C3435T and the grading of neutropenia was observed (p>0.05). However, we observed the trend of declining absolute neutrophil count with higher chemotherapy cycles (Table 3) and also there was no any significant deviation of alelle and genotype frequency from Hardy-Weinberg Equilibrium (p>0.05).
Table 3. Trend of declining absolute neutrophil count
DISCUSSION
From this study we can find various ethnic groups including Bataknese, Javanese, Minangkabau, and Acehnese. This study was relevant with observation that said breast cancer incidence was varied among different race/ethnicities with white women having a tendency to get breast cancer compared to African-American women.11 Difference between incidence rates and survival rates from different ethnic groups was a result from cultural and environmental factors in terms of unpreventabe risk factor, exposure to carcinogenic agents, socioeconomic status, and genetics. Unpreventable risk factors include age during first menarche, age during menopause, and age during first birth. These factors were associated with the duration of estrogen exposure.12
From this study, the breast cancer patients were in 44–51 years old group. This result was relevant with recent study with the most of breast cancer patients were in range 44–55 years of age.13 From this study, the subjects between age 45–68 years old and overweight were 22 person (30.56%), and 10 person was obese (13.89%) (data not shown). Post-menopausal obese women have a higher risk for breast cancer due to aromatase activity which converts androstenendion to estrogen.12 This study also showed that the most frequent type of breast cancer based on histopathology was invasive ductal carcinoma. This result was relevant with prior study which showed that invasive ductal carcinoma was the most common type found.14
In this study, ABCB1 C3435T polymorphism did not have an association with neutropenia. This result was relevant with prior study for 121 breast cancer patient which received paclitaxel monotherapy that showed there was no association between polymorphism of ABCB1 C3435T with neutropenic event.15 Another study also showed for 1,012 breast cancer patients receiving antracycline, 5-fluorouracyl (5-FU) and cyclophosphamide (CAF) for 3–6 cycles also showed there was no association between ABCB1 polymorphism with neutropenia grade III-IV.3
This result was contradictive with study in 58 cancer patients with docetaxel monotherapy that there was an association between polymorphism of ABCB1 C3435T with grade III neutropenia with TT homozygous cancer patients experiencing grade III neutropenia.16 Study in 18 patients showed that there was a decline in absolute neutrophil count up to 80% in patients that have homozygous TT after administration of paclitaxel monotherapy.8
Another study also showed that polymorphism of ABCB1 C3435T have an asociation with grade III and IV neutropenia in acute lymphoblastic leukemia in children who received chemotherapy.17 Several studies also showed that there were up to two times of declining of P-gp level in individual who carries homozygous variant TT of ABCB1 C3435T.18
The result which showed no association between ABCB1 C3435T polymorphism and neutropenia can be reasoned by two factors, administration of doxorubicin-paclitaxel given by standard doses and other genetic factors which influence the function of P-gp which encoded by ABCB1 gene.19
In this study, patients were given standard doses of doxorubicin of 50 mg/m2 and paclitaxel of 175 mg/m2. This result was relevant with the study in 112 breast cancer patients that showed increase of severity of neutropenia, trombocytopenia and anemia along with the increase of doxorubicin doses. Patients who received more than 60 mg/m2 in this case 75 mg/m2 and, 90 mg/m2 have a tendency to get granulocyte-colony stimulating factor (G-CSF) or prophylaxis antibiotic. Additional toxicity that can happen after paclitaxel administration (doses of 175 mg/m2) can generally be tolerated by patients. Granulocytopenia occurs in 62% patients receiving doxorubicin-cyclophosphamide and just 16% of patients with paclitaxel.20
The prior study also supports our findings, in 275 breast cancer patients that show that was an increase of febrile neutropenia of about 32% with 60 mg/m2 doxorubicin, and 175 mg/m2 paclitaxel. The other study also showed that combination of doxorubicin-paclitaxel have a better response rate (47%) than doxorubicin monotherapy (36%) or paclitaxel monotherapy (34%).21 Another study reported that neutropenia grade III-IV did not happen in 112 breast cancer patients although this may be due to subjects were also give filgastrim, a G-CSF, along the doxorubicinpaclitaxel regimen.22 Several studies showed that the administration of doxorubicin-paclitaxel with appropriate standard doses proved effective and can be tolerated by patients.
The second factor is that there was an association between ABCB1 C3435T polymorphism with several other SNP in ABCB1 gene that can influence the expression of P-gp. Based on the latest study, we know that there are 50 known SNPs in ABCB1 gene. Collection of several SNPs that are linked is known as haplotype. The most common haplotype is the combination of C3435T with G2677T and C1236T. Haploptype might be responsible for variation of P-gp function compared to that of just one SNP.4,7
This study is also supported by other study which showed that there was a difference of P-gp function caused by ABCB1 haplotype.23 Anoher study showed that combination of C3435T with G2677T and C1236T polymorphisms can cause a less functional P-gp.24 In C3435T polymorphism, the change in nucleotide (C to T) can cause ribosomal delay and leads to ribosome stalling during translation. Significant delay of translation will disrupt interaction with chaperone protein and resulting in P-gp protein production with a slightly different structure.7 In this study, we also found trend of absolute neutrophil declining with more chemotherapy cycles, the prior study also supports our findings, which explained the risk of neutropenia in later cycles of chemotherapy and the administration of growth factor can reduce the severity of neutropenia in the later cycles of chemotherapy.25
The limitations of this study were the other hematologic parameters from complete blood counts (e.g. leucocyte, platelet) were not taken into account because we only focused on the effect of chemotherapy to neutrophil counts. As far as we were concerned, the other hematologic parameter did not disturb the result of this study because they did not impact to neutrophil count directly whereas the other confounding factors that could intrude neutrophil counts, such as patients who had infection, or had taken drugs that can interfere the counting, were already excluded in this study.
In conclusion, there was no association of ABCB1 C3435T polymorphism with neutropenia grading, further studies need to be done to analyze the impact of haplotype on neutropenic episodes in breast cancer patients receiving doxorubicintaxane regimen.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
We would like to thank Mardiah Nasution and Indra Wahyudi for their excellent technical assistance, Donny Nauphar for his helpful review of the manuscript.
REFERENCES
- World Health Association [Internet]. Breast cancer: prevention and control. [updated 2013 May 05, cited 2013 Aug 12]. Available from: http://www.who.int/ cancer/detection/breastcancer/en/
- Sistem Informasi Rumah Sakit [Internet]. Prevalensi kanker payudara. [updated 2011 Des 12, cited 2013 Feb 8]. Available from: http://sirs.buk.depkes.go.id/sirs. Indonesian
- Vulsteke C, Lambrechts D, Dieudonné A, Hatse S, Brouwers B, van Brussel T, et al. Genetic variability in the multidrug resistance associated protein-1 (ABCC1/ MRP1) predicts hematological toxicity in breast cancer patients receiving (neo-)adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC). Ann Oncol. 2013;24(6):1513–25.
- Huang Y. Pharmacogenetics/genomics of membrane transporters in cancer chemotherapy. Cancer Metastasis Rev. 2007;26(1):183–201.
- Franke RM, Gardner ER, Sparreboom A. Pharmacogenetics of drug transporters. Curr Pharm Des. 2010;16(2):220–30.
- Thorn C, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–6.
- Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794(5):860–71.
- Sissung TM, Mross K, Steinberg SM, Behringer D, Figg WD, Sparreboom A, et al. Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer. 2006;42:2893–6.
- Wang D, Johnson AD, Papp AC, Kroetz DL, Sadeé W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C> T affects mRNA stability. Pharmacogenet Genomics. 2005;15(10):693–704.
- National Cancer Institute [Internet]. Common terminology criteria for adverse events v4.0 (CTCAE). [update June 2010; cited 2013 Sep]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/ CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Morris CR, Epstein J, Nassere K, Hofer BM, Rico J, Bates JH, et al. Trends in cancer incidence, mortality, risk factors, and health behaviors in California. Sacramento: Cancer Surveillance Section; 2010. p. 17–9.
- ecommons.cornell.edu [Internet]. Breast cancer in women from different racial/ethnic groups. [update Apr 2003, cited 2012 Des]. p. 1–5.
- Norsa’adah B, Rusli BN, Imran AK, Naing I, Winn T. Risk factors of breast cancer in women in Kelantan, Malaysia. Singapore Med J. 2005;46(12):698–705.
- Albrektsen G, Heuch I, Thoresen SØ. Histological type and grade of breast cancer tumors by parity, age at birth, and time since birth: a register-based study in Norway. BMC Cancer. 2010;10:226.
- Chang H, Rha SY, Jeung HC, Im C, Ahn JB, Kwon WS, et al. Association of the ABCB1 gene polymorphisms 2677G> T/A and 3435C> T with clinical outcomes of paclitaxel monotherapy in metastatic breast cancer patients. Ann Oncol. 2009;20(2):272–7.
- Tran A, Jullien V, Alexandre J, Rey E, Rabillon F, Girre V, et al. Pharmacogenetics and and toxicity of docetaxel: role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79(6):570–80.
- Erdélyi DJ, Kámory E, Zalka A, Semsei AF, Csókay B, Andrikovics H, et al. The role of ABC-transporter gene polymorphisms in chemotherapy induced immunosuppression, a retrospective study in childhood acute lymphoblastic leukaemia. Cell Immunol. 2006;244(2):121–4.
- Franke R, Gardner E, Sparreboom A. Pharmacogenetics of drug transporters. Curr Pharm Des. 2010;16(2):220–30.
- Kudzi W, Dodoo AN, Mills JJ. Genetic polymorphisms in MDR1, CYP3A4 and CYP3A5 genes in a Ghanaian population: a plausible explanation for altered metabolism of ivermectin in humans?. BMC Med Genet. 2010;11:111.
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83.
- Sledge G. First-line chemotherapy for advancer breast cancer. J Moffit Cancer Cent. 1999;6(5):4–7.
- Schwartz J, Domchek SM, Hwang WT, Fox K. Evaluation of anemia, neutropenia and skin toxicities in standard or dose-dense doxorubicin/cyclophosphamide (AC)- paclitaxel or docetaxel adjuvant chemotherapy in breast cancer. Ann Oncol. 2005;16(2):247–52.
- Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, et al. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13(8):481–94.
- Kimchi-Sarfaty C, Oh JM, Kim I, Sauna ZE, Calcagno AM, Ambudkar SV. A “Silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315(5811):525–8.
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–37.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id