
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Impact of pregnancy-induced hypertension on fetal growth
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i2.1381 Med J Indones. 2016;25:104–11
Received: February 11, 2016
Accepted: June 06, 2016
Author affiliation:
1 Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
2 Department of Obstetrics and Gynecology Ende hospital, Ende, East Nusa Tenggara, Indonesia
Corresponding author:
Raymond Surya
E-mail: raymond_s130291@yahoo.co.id
Background
Pregnancy-induced hypertension (PIH) is still a major cause of maternal and infant morbidity and mortality worldwide. The aim of this study to investigate the impact of PIH on fetal growth.
Methods
A longitudinal cross-sectional study was conducted by 2,076 obstetric patients registered in the book of delivery emergency room BLUD RSUD Ende/ Ende hospital from September 1st 2014 to August 31st 2015. Pregnancy-induced hypertension was classified into gestational hypertension, preeclampsia, and severe preeclampsia. Categorical comparative chi-square continued by logistic regression analysis were performed to examine the effect of PIH to infants’ growth outcome.
Results
Women with preeclampsia had higher number of preterm delivery (26.7%). Infants born from preeclamptic women had lower birth weight (median 2,575 gram; p<0.001), birth length (median 49 cm; p<0.001), and also head circumference (median 32 cm; p<0.001). Severe preeclampsia contributed statistically significance to SGA (OR=1.90; 95% CI=1.20-3.01; adjusted OR=1.91; 95% CI=1.20-3.01) and LGA (OR=2.70; 95% CI=1.00-7.29; adjusted OR=2.92; 95% CI=1.07-8.00). Based on birth weight independent of gestational age, severe preeclampsia had an impact to VLBW (OR=11.45; 95% CI=2.77-47.38; adjusted OR=8.68; 95% CI=1.57-48.04) and LBW (OR=6.57; 95% CI=4.01-10.79; adjusted OR=5.71; 95% CI=3.33-9.78) where it showed statistical significance.
Conclusion
PIH women who had SGA or VLBL or LBW infants were caused by the hypoperfusion model as the pathogenesis of preeclampsia. Meanwhile, LGA infants born by preeclamptic women were due to the compensation of the decrease from uteroplacental perfusion or other diseases such as obese mother or gestational.diabetes mellitus.
Keywords
fetal growth, pregnancy-induced hypertension
Pregnancy-induced hypertension (PIH), especially preeclampsia, is still a major cause of maternal and infant morbidity and mortality worldwide.1 The prevalence in worldwide ranges from 3% to 8% of all pregnancies; meanwhile in USA, it affects from 2% to 5% of pregnancies.2 Based on Riset Kesehatan Dasar 2007 in Indonesia, PIH is one of three main causes of maternal morbidity and mortality of which its prevalence was around 12.7%.3 Unfortunately, several studies still cannot reveal the etiology and pathogenesis of preeclampsia.1 Many theories indicate that intrauterine growth restriction which impacts to low birth weight is the result of the decrease of placental perfusion.4
In most cases of preeclampsia, the pregnant women have no history of hypertension. Several risk factors predispose women to preeclampsia including nulliparity, age 40 years or older, obesity (body mass index >35 kg/m2), a pregnancy interval of more than 10 years, previous history of preeclampsia or gestational hypertension, preexisting renal disease, and multiple pregnancies. Genetic factors are at least partially responsible whether a maternal or a paternal family history of preeclampsia will increase the risk of developing it.5
Fetal growth consists of several variables, namely birth weight (BW), birth length (BL), and head circumference (HC). It is determined by both duration of gestation and complication from maternal condition, especially preeclampsia. The incidence of preterm birth increases significantly due to preeclampsia. We know that the best solution for preeclampsia is prompt delivery in order to save the mother. Apart from that, intrauterine growth restriction (IUGR) and hypertensive disorders have been considered to reflect the same uteroplacental insufficiency and used to quantify the severity of preeclamptic state.6 Therefore, our objective is to know the impact of PIH on fetal growth through components stated above by comparing with the normotensive mother.
METHODS
The data collection for this study originated from the register book of delivery emergency room Badan Layanan Umum Daerah (BLUD) RSUD Ende / Ende hospital, Ende, East Nusa Tenggara, Indonesia. Confidentiality of subjects’ identities were guaranteed. Only obstetric data from September 2014 to August 2015 was recorded. There were 32 components recorded in this book relating to demographic information, referral status, early diagnosis, methode of delivery, birth outcome, complication of neonates, and last diagnosis.
We analyzed all completed data without considering any sign of life. The exclusion criteria were women with pre-existing (chronic) hypertension (17 patients), multiple pregnancies (31 patients), history of diabetes (two patients), cardiovascular disease (one patient), and also premature rupture of membrane (291 patients). We believed that these conditions as cofounding variables which could influence the pathogenesis of preeclampsia and birth outcomes. The cases of abortion, mola hydatidosa, ectopic pregnancy, blighted ovum, and not delivering were 194, 10, 3, 2, and 141 cases; respectively. After eliminating 70 cases with missing information, we analyzed 1,314 deliveries.
PIH was classified as gestational hypertension, preeclampsia, and severe preeclampsia. An office (or in-hospital) systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg based on the average of at least two measurements, taken at least 15 minutes apart, using the same arm without proteinuria after 20th week of gestation was called by gestational hypertension. Meanwhile, preeclampsia was diagnosed by gestational hypertension with one or more of the following new proteinuria, or one or more adverse conditions, or one or more severe complications. Significant proteinuria was defined as ≥0.3 g/d in a complete 24-hour urine collection or ≥30 mg/mmol urinary creatinine in a random urine sample or urinary dipstick proteinuria ≥1+. Some adverse conditions consisted of maternal symptoms, signs, and abnormal laboratory results, and abnormal fetal monitoring results impacts to the maternal and also the fetal condition. Actually, severe preeclampsia was still in the scope of preeclampsia, whereas it had one or more severe complications. Severe complications that warrant delivery consisted of eclampsia, retinal detachment, Glasgow coma scale (GCS) <13, stroke, uncontrolled severe hypertension, oxygen saturation <90%, myocardial ischemia, platelet count <50x109/L, acute kidney injury, hepatic dysfunction, placental abruption, and stillbirth.7
Gestational age was defined through last menstrual period dates recorded in Buku Kesehatan Ibu dan Anak (Mother and Child Health Book), physical examination, and if available, it was supported by the result of first-trimester or early second-trimester ultrasonography. Birth weight was classified into two categories, namely for a specific gestational age (GA) and independent of GA. Birth weight based on specific GA was divided into small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational age (LGA). SGA was defined as BW <10th percentile of expected weight for GA, BW between 10th and 90th percentile of expected weight for GA was called as AGA, and LGA was defined as BW >90th percentile of expected weight for GA. Birth weight independent of GA was classified into extremely low birth weight (ELBW) where BW <1,000 gram, very low birth weight (VLBW) where BW <1,500 gram, low birth weight (LBW) where BW was from 1,500 to 2,500 gram, normal weight was between 2,500-4,000 gram, and high birth weight (HBW) where BW >4,000 gram. Meanwhile, BL and HC were not classified so that they were analyzed in numerical variables.
We described the potential confounding variables which had an association to exposure and outcome based on previous literature. Some those variables in this study were maternal age, parity, and gestational age of delivery.
The rates, odds ratios (ORs), and 95% confidence interval compared binary outcomes (SGA, LGA, ELBW, LBW, VLBW, and HBW) between groups of PIH and normotensive women. Statistical analysis was performed using chisquare for the categorical variables. Analysis of variance (ANOVA) was performed to compare maternal age, GA, preterm delivery, parity, referral status, methods of delivery, neonates sex, BW, BL, and HC among the groups with gestational hypertension, preeclampsia, severe preeclampsia to the patients with normal blood pressure. After doing that univariate analysis, we included maternal age, GA, and parity as cofounding variables in the multivariate logistic regression through the adjusted ORs and 95% CI as the indicator. All p-values were two tailed and the significance level selected was <0.05. All statistical analyses were performed with SPSS 23.0 for Windows.
RESULTS
During this one year period, there were 2,076 cases were registered in the book of delivery emergency room BLUD RSUD Ende, of which 187 women (9%) were PIH. Of these 187 women, 58 (31.0%) had gestational hypertension, 39 (20.9%) had preeclampsia, and 90 (48.1%) had severe preeclampsia. The mean maternal age at delivery was 30.3±6.4 years. Most of women in this study were nulliparous (40.5%). The most referral status of women delivering in hospital came from primary health care (50.3%). The characteristic of patients with PIH was shown in Table 1. They were divided into normotensive, gestational hypertension, preeclampsia, and severe preeclamptic women.
Table 1. Demographic and reproductive characteristics of patients in BLUD RSUD Ende, 2014–2015
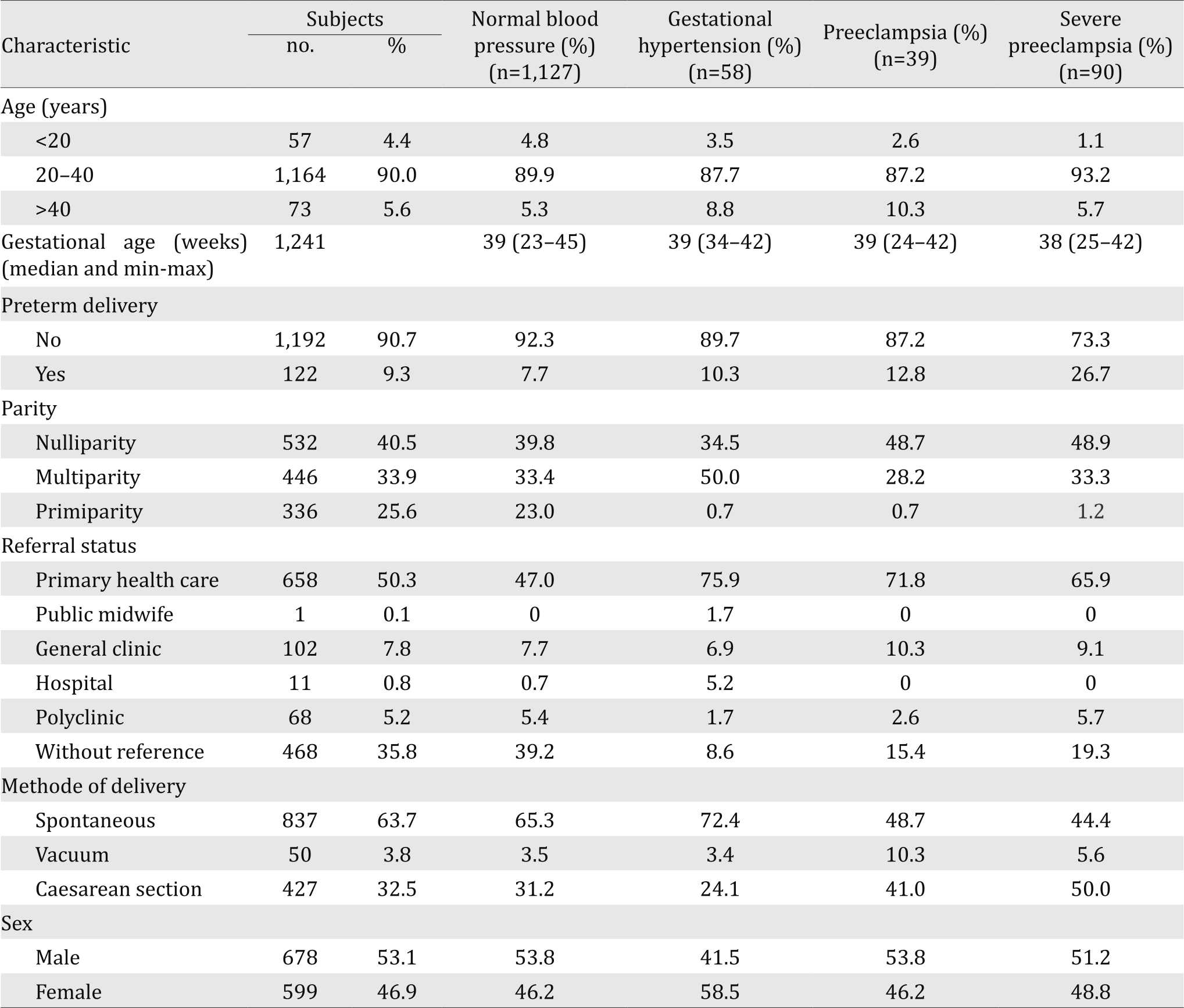
Table 2 summarizes the effects of PIH on BW for a specific GA after analyzing with multivariate logistic regression. The rate of SGA was higher in PIH women than normotensive women. Women with severe preeclampsia had 1.90 times higher (95% CI=1.20-3.01) to have SGA infants compared to normotensive women. The incidence of SGA was not statistically significant in gestational hypertension and preeclamptic women. The highest rate of LGA infants was in severe preeclamptic women (10.6%). There was statistically significant (OR=2.70; 95% CI=1.00-7.29; p=0.042).
Table 2. Birth weight for a specific gestational age in PIH women, univariate and multivariate logistic regression in BLUD RSUD Ende, 2014–2015
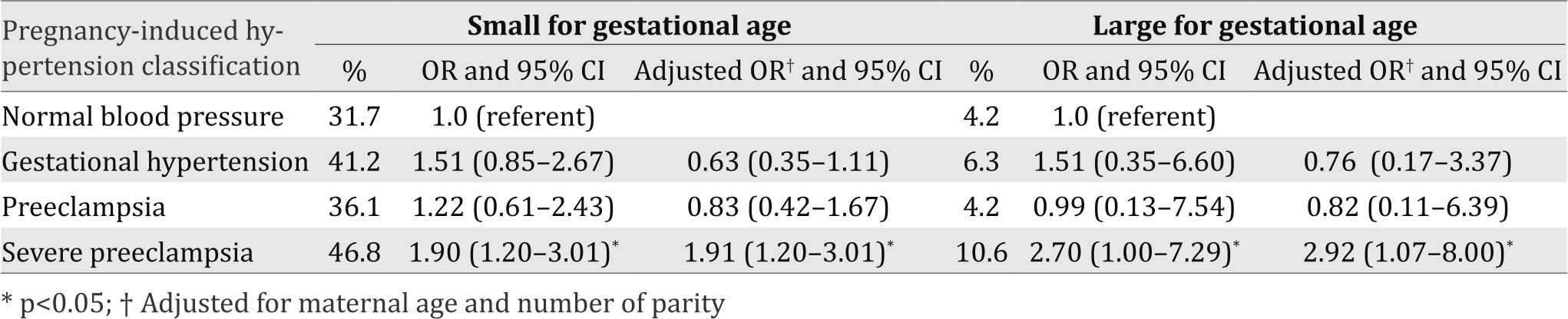
Table 3 describes the impact of PIH on BW independent of GA. The highest rate of ELBW was in severe preeclampsia (8.7%). The rate of LBW in normotensive women was 10.7% and it increased in PIH women where 22.6% in gestational hypertension, 14.3% in preeclampsia, and 44.0% in severe preeclampsia. Only gestational hypertension (p=0.007) and severe preeclampsia (p<0.001) showed statistical significance with OR=2.45 (95% CI=1.25-4.80) and 6.57 (95% CI=4.01-10.79). Preeclamptic and severe preeclamptic women had higher rate of HBW infants (3.2% and 4.5%, respectively) than normotensive women (1.2%). Unfortunately, there was no significance in statistic both in preeclampsia and severe preeclampsia to HBW (p=0.333 and p =0.065, respectively).
Table 3. Birth weight independent of gestational age in PIH women, univariate and multivariate logistic regression in BLUD RSUD Ende, 2014-2015
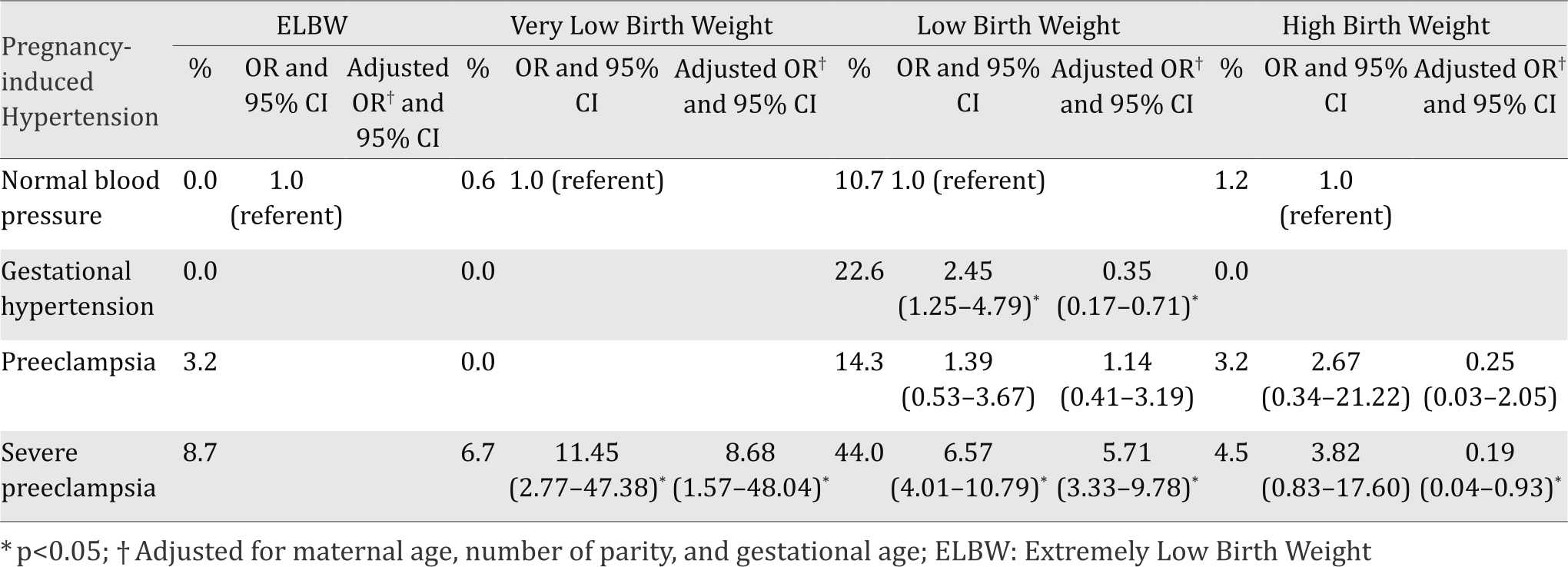
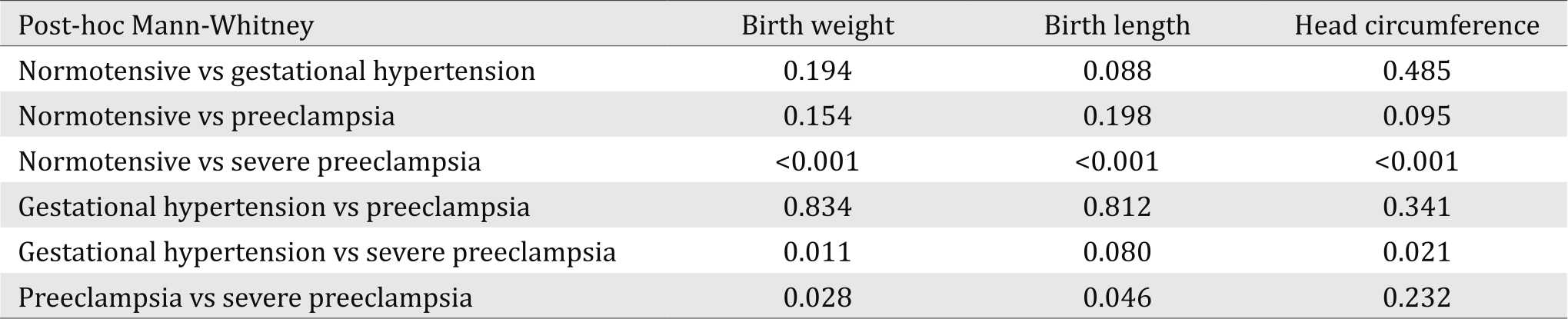
Table 4 shows the difference of BW, BL, and HC in PIH women. For the outcome of infants, infants born from preeclamptic women were lower BW (median 2,575 grams), BL (median 49 cm), and also HC (median 32 cm). In analysis of variance using non-parametric test (Kruskal-Wallis), it described there was statistical significance among PIH women group in BW (p<0.001), BL (p<0.001), and HC (p<0.001). Table 5 concludes the post hoc analysis to know the difference among PIH group which made significant in statistic. Normotensive women had statistical significance compared to severe preeclamptic women in BW (p<0.001), BL (p<0.001), and HC (p<0.001). Meanwhile, gestational hypertension vs severe preeclamptic women had significance in statistic for BW (p=0.011). Preeclamptic and severe preeclamptic women had significant difference in BW (p=0.028) and BL (p=0.046).
Table 4. Birth weight, birth length, and head circumference in PIH women in BLUD RSUD Ende, 2014–2015
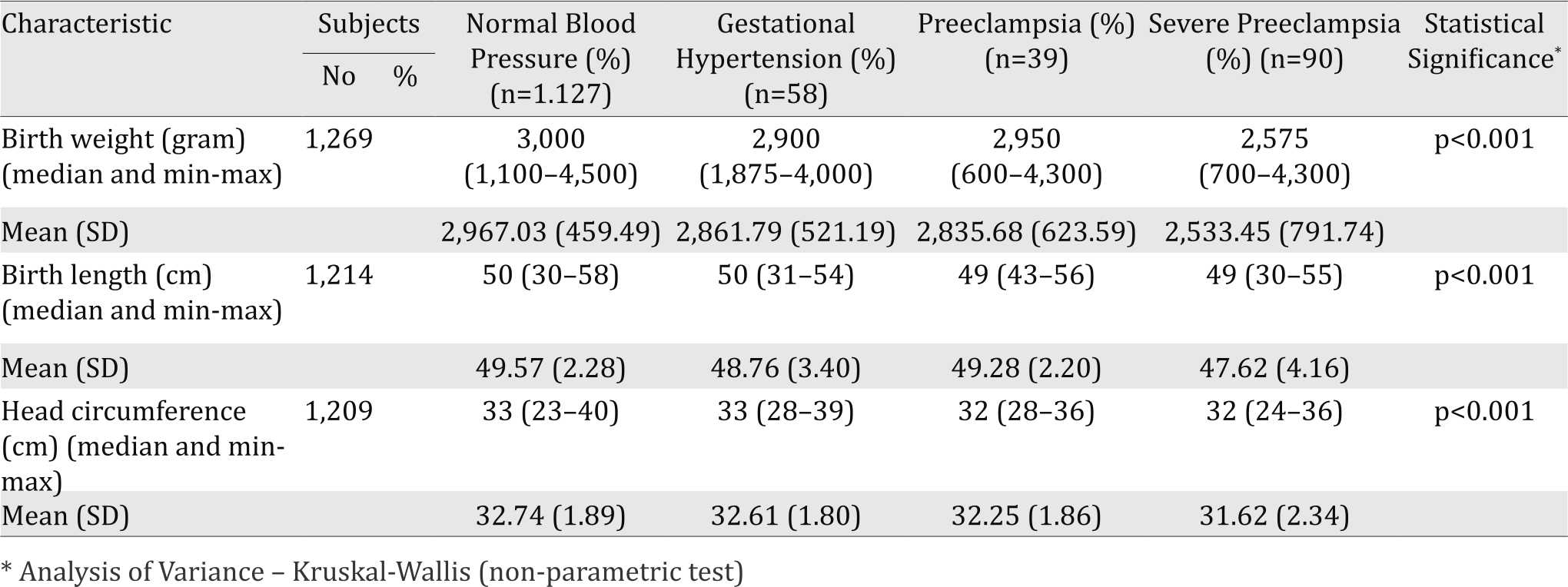
Table 5. Post-hoc analysis to determine the difference among PIH women group in BLUD RSUD Ende, 2014–2015
DISCUSSION
To know the impact of PIH as an independent risk factor on fetal growth, we had to minimize the bias factors. One of the bias factors is preexisting (chronic) hypertension. Based on study by Haelterman et al8, the risk of intrauterine growth restriction was increased in the condition of chronic hypertension. Chronic hypertensive disorders or chronic kidney disease can influence hypertension; therefore, we excluded all of those and classified PIH into three groups based on Magee et al7 in SOGC Clinical Practice Guideline namely gestational hypertension, preeclampsia, and severe preeclampsia. The incidence of PIH in this study was 14.2%, which is higher than a study by Xiong et al9 (9.0%).
Mothers with preeclampsia usually have preterm delivery or shortened duration of GA because early delivery is the only effective treatment for patients with PIH. As shown in Table 1, the shortest GA was in severe preeclamptic women whereas the median was 38 weeks (25-42 weeks). This result is similar to the study by Fatemeh et al10, which showed that hypertensive nulliparous women had shortened gestational age (37.37±2.25 weeks) compared to normotensive nulliparous women (38.81±1.71 weeks). Apart from that, the study performed by Buchbinder et al11 concluded that preterm delivery is only associated with severe hypertension and proteinuria does not affect the outcomes. Macdonald-Wallis et al12 in their study stated that blood pressure change between 30 and 36 weeks may be associated with the timing of spontaneous labor. This result is similar to a multinational study of >8,000 women that a greater increase in blood pressure was correlation to the greater risk of spontaneous preterm birth.13 Early delivery increases the rate of caesarean section as one of the ways to end the pregnancy increased in accordance with the severity of PIH. The study by Gofton et al.14 showed that PIH women had obstetrical intervention rates much higher than normotensive ones. The obstetrical intervention here was the induction and caesarean delivery rates. Increased rate of caesarean section among PIH women was reported in this study and the highest rate was among severe preeclamptic women (50.0%).
Among 187 PIH cases in this study, we found SGA infants in 71 cases (37.97%), AGA infants in 95 cases (50.80%), and LGA infants in eight cases (4.28%). In the classification of BW independent of GA in PIH women, ELBW infants were found in five (2.67%), VLBW infants in three (1.60%), LBW infants in 50 (26.74%), normal weight infants in 113 (60.43%), and HBW infants in three (1.60%). Meanwhile, 1,127 cases of women with normal blood pressure group as a comparison in this study, most of infants were AGA (64.42%) and normal weight (85.36%). Both SGA and LGA infants were lower than in PIH group (29.90% and 2.84%, respectively). Also, the ELBW, VLBW, LBW, and HBW infants were lower than in case group. Patients with severe preeclampsia had 1.90 (adjusted OR=1.91) times higher to have SGA infants. Based on the BW independent of GA, severe preeclampsia increased the risk of VLBW and LBW infants and there were statistical significancies. The result is similar to study published by Xiong et al9 and Eskenazi et al.15 One of the reasons that more severe PIH had SGA or VLBW and LBW infants was about hypoperfusion model as pathogenesis of preeclampsia. Hypertension or preeclampsia lowered the uteroplacental perfusion by contracting the plasma volume.16 It reduced transfer of oxygen and nutrients to the developing fetus. The hypoxic placenta in turn releases antiangiogenic factors into maternal circulation which are hypothesized to invoke the maternal inflammatory response including endothelial dysfunction and increased blood pressure.17 Apart from that, the literature states that fetal hypoxia and growth restriction are caused by abnormal placentation. Meanwhile, proteinuria is a sign for the vascular damage which contributes to this condition.18 However, there still debates between the effect of intrauterine growth restriction and neonatal morbidity and mortality. Another study conducted by von Dadelszen et al.19 reported that there was an association between hypertension in women and condition of fetus at birth, also the survival of neonates to discharge from neonatal intensive care unit.
Women with severe preeclampsia had OR=2.70 (95% CI=1.00-7.29) to deliver LGA infants and it was statistically significant. Apart from that, based on BW independent of GA, severe preeclamptic women also had 3.82 times higher to deliver HBW infants, although in statistic, it was not significant. The result is similar to study by Xiong et al.20 The lack of nutrient on fetus increased the resistance of mother peripheral circulation which finally ended in pregnancy-induced hypertension. The raising of dehydroisoandrosterone sulphate as an indicator of placental perfusion was an effort of body to increase the uteroplacental perfusion in some PIH women.21 However, some patients with PIH deliver larger infants because the incidence of preeclampsia occurs later in pregnancy. The short duration from the decrease of uteroplacental perfusion develop the body’s compensation to this condition by reversing the effect. Apart from that, proteinuria as an indicator of preeclampsia can function as “protective” factor to keep the uteroplacental blood flow19,22 or other diseases, such as obese mother or gestational diabetes mellitus of which the data are not available. Therefore, a long longitudinal study should be conducted to know the role of proteinuria in pregnancy-induced hypertension to uteroplacental blood flow.
According to study published by Xiong et al9, Naeye22, we found that there was no difference in mean birth weight between the various PIH and normotensive one; however, the mean birth weight was the lowest in severe preeclampsia group (2,533.45±791.74 grams vs 2,967.03±459.49 in normotensive group). Apart from that, both the mean of birth length and also head circumference were the lowest in severe preeclampsia (47.62±4.16 cm and 31.62±2.34 cm respectively). The result was similar to the study by Lim et al.23 which declared that peripheral systolic blood pressure would decrease birth weight, birth length, head circumference, and placental weight (all p<0.05). Each 1 SD (11.1 mmHg) increase of peripheral systolic blood was inversely associated with birth weight (-35.56 g; 95% CI=-66.57 to -4.54), birth length (-0.16 cm; 95% CI=-0.32-0.01), head circumference (-0.09 cm; 95% CI=-0.19-0.02), and placental weight (-8.78 g; 95% CI=-18.74-1.19).
The limitation of our study was there were no data about maternal body mass index (BMI), maternal smoking, previous conditions, and numbers of antenatal visit. In summary, the findings in this study depict the lack of a causal relationship between hypertensive disorders of pregnancy and the neonates’ growth outcome. Therefore, future studies should be established to know the causal relationship how far the contribution of PIH influences the growth outcome of infants so that prevention and therapy can be given based on the pathophysiology of it.
PIH women who had SGA or VLBL or LBW infants were caused by the hypoperfusion model as the pathogenesis of preeclampsia. Meanwhile, LGA infants born by preeclamptic women were due to the compensation of the decrease from uteroplacental perfusion.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
Thank you to Ende hospital and all midwives who help the writer to the data preparation; also to Albert Sedjahtera, Angela Christina, Fransisca, Handayan Hutabarat, Tissy Fabiola, Jesslyn Gunardi, Fidini Hayati, and the other internship doctors who had supported for the manuscript arrangement.
REFERENCES
- Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447-51.
- Hermes W, Van Kesteren F, De Groot CJ. Preeclampsia and cardiovascular risk. Minerva Gynecol. 2012;64(4):281-92.
- Sirait AM. Prevalensi hipertensi pada kehamilan di Indonesia dan berbagai faktor yang berhubungan (Riset Kesehatan Dasar 2007). Buletin Penelitian Sistem Kesehatan. 2012;15(2):103-9. Indonesian.
- National Heart, Lung, and Blood Institute National High Blood Pressure Education Program. National high blood pressure education program working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183(1):S1-22.
- Lin S, Leonard D, Co MA, Mukhopadhyay D, Giri B, Perger L, et al. Pre-eclampsia has an adverse impact on maternal and fetal health. Transl Res. 2015;165(4):449-63.
- Grisaru-Granovsky S, Halevy T, Eidelman A, Elstein D, Samueloff A. Hypertensive disorders of pregnancy and the small for gestational age neonate: not a simple relationship. Am J Obstet Gynecol. 2007;196(4):335.e1-5.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-38.
- Haelterman E, Bréart G, Paris-Llado J, Dramaix M, Tchobrousky C. Effect of uncomplicated chronic hypertension on the risk of small-for-gestational age birth. Am J Epidemiol. 1997;145(8):689-95.
- Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn-Cook C, et al. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999;180(1Pt1):207-13.
- Fatemeh T, Marziyeh G, Nayereh G, Anahita G, Samira T. Maternal and perinatal outcome in nulliparious women complicated with pregnancy hypertension. J Pak Med Assoc. 2010;60(9):707-10.
- Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, et al. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66-71.
- Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension. 2014;64(1):36-44.
- Zhang J, Villar J, Sun W, Merialdi M, Abdel-Aleem H, Mathai M, et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197(2):162.e1-6.
- Gofton EN, Capewell V, Natale R, Gratton RJ. Obstetrical intervention rates and maternal and neonatal outcomes of women with gestational hypertension. Am J Obstet Gynecol. 2001;185(4):798-803.
- Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol. 1993;169(5):1112-8.
- Gallery ED, Hunyor SN, Györy AZ. Plasma volume contraction: a significant factor in both pregnancyassociated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med. 1979;48(192):593-602.
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592-4.
- Ferrazzani S, Caruso A, De Carolis S, Martino IV, Mancuso S. Proteinuria and outcome of 444 pregnancies complicated by hypertension. Am J Obstet Gynecol. 1990;162(2):366-71.
- von Dadelszen P, Magee LA, Taylor EL, Muir JC, Stewart SD, Sherman P, et al. Maternal hypertension and neonatal outcome among small for gestational age infants. Obstet Gynecol. 2005;106(2):335-9.
- Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for age. Am J Obstet Gynecol. 2000;183(1):148-55.
- Chen XK, Wen SW, Smith G, Yang Q, Walker M. Pregnancy-induced hypertension and infant mortality: roles of birthweight centiles and gestational age. BJOG. 2007;114(1):24-31.
- Naeye RL. Maternal blood pressure and fetal growth. Am J Obstet Gynecol. 1981;141(7):780-7.
- Lim WY, Lee YS, Tan CS, Kwek K, Chong YS, Gluckman PD, et al. The association between maternal blood pressures and offspring size at birth in Southeast Asian women. BMC Pregnancy and Childbirth. 2014;14:403.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id