Review Article
Management of overactive bladder review: the role of percutaneous tibial nerve stimulation
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i4.1385 Med J Indones. 2016;25:245–54
Received: February 14, 2016
Accepted: September 24, 2016
Author affiliation:
Department of Urology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Corresponding author:
Harrina E. Rahardjo
E-mail: harrinaerlianti@yahoo.com
Overactive bladder (OAB) is a common condition that is experienced by around 455 million people (11% of the world population) and associated with significant impact in patients’ quality of life. The first line treatments of OAB are conservative treatment and anti-muscarinic medication. For the refractory OAB patients, the treatment options available are surgical therapy, electrical stimulation, and botulinum toxin injection. Among them, percutaneous tibial nerve stimulation (PTNS) is a minimally invasive option that aims to stimulate sacral nerve plexus, a group of nerve that is responsible for regulation of bladder function. After its approval by food and drug administration (FDA) in 2007, PTNS revealed considerable promise in OAB management. In this review, several non-comparative and comparative studies comparing PTNS with sham procedure, anti-muscarinic therapy, and multimodal therapy combining PTNS and anti-muscarinic had supportive data to this consideration.
Keywords
electrical stimulation, overactive bladder, percutaneous tibial nerve stimulation
Overactive bladder (OAB) is a common disorder and pervasive problem throughout all aspects of life that causes considerable effects on patients’ quality of life.1 This term specifically refers to a type of voiding dysfunction that consists of urgency, with or without urgency incontinence, without other pathology. Usually, it is accompanied with frequency and nocturia.2
It is estimated that there are around 455 million people (11% of the world population) having experienced OAB symptoms during their life. The reported OAB prevalence is dominated by females varying from 7.7% to 31.3% compared with males from 10.2% to 17.4%.2–4 In Indonesia, the prevalence of OAB is 4.1% and 1.6% for wet and dry OAB, respectively. In pediatric population, the majority urinary incontinence (UI) is wet OAB (2.1%), similar to in adult and geriatric population (4.6%).5
As previously stated, OAB can give significant impact to quality of life, involving physical, sexual function, psychological and social relation.1,6 Sumardi et al5 found that UI, including OAB, impaired family life (25.3%), school/job performance (23.7%), and sexual relationship (13.6%). Therefore, the proper choice of treatment is necessary to overcome OAB problem. First line treatments of OAB are conservative treatment combined with anti-muscarinic medication. For those resistant to the first line option, the treatment options available are surgical therapy, electrical stimulation and botulinum toxin injection.7
Etiology
Generally, the etiology of OAB is unknown, but it can be related to neurologic problems, as they may affect bladder innervation. The most common neurologic diseases mentioned are multiple sclerosis and Parkinson’s disease. Increased lower urinary tract symptoms (LUTS) is also related to aging with decrease on brain function and inhibitory innervation. There is an age-related decrease of neuronal acetylcholine release and increase of stretch related acetylcholine from urothelium non-neuronal cells that may cause detrusor contraction. Another factor that is important to be considered in OAB is bladder outlet obstruction (BOO) with its impact in detrusor overactivity. The mechanism is hypersensitivity to acetylcholine and denervation of cholinergic detrusor. Moreover, there are several effects caused by prolonged BOO such as nerve growth factor production increase, neuronal enlargement, and spinal micturition reflex enhancement.7,8 A study by Steers9 showed that there were increases of bladder’s nerve growth factor and alteration of neuron excitability related to increased nerve growth factor access, in patients with OAB and benign prostatic hyperplasia.
Neurophysiology of bladder function
The regulation of bladder is controlled by sacral nerve plexus, located at the base of the spine.6 In order to maintain continence, the normal detrusor function is regulated in sacral balance under the control of sympathetic and parasympathetic nerves. Usually, the sympathetic tone plays a role in storage of urine, while the parasympathetic nerve controls the emptying of the bladder.10 The innervation of the bladder can be seen in Figure 1.
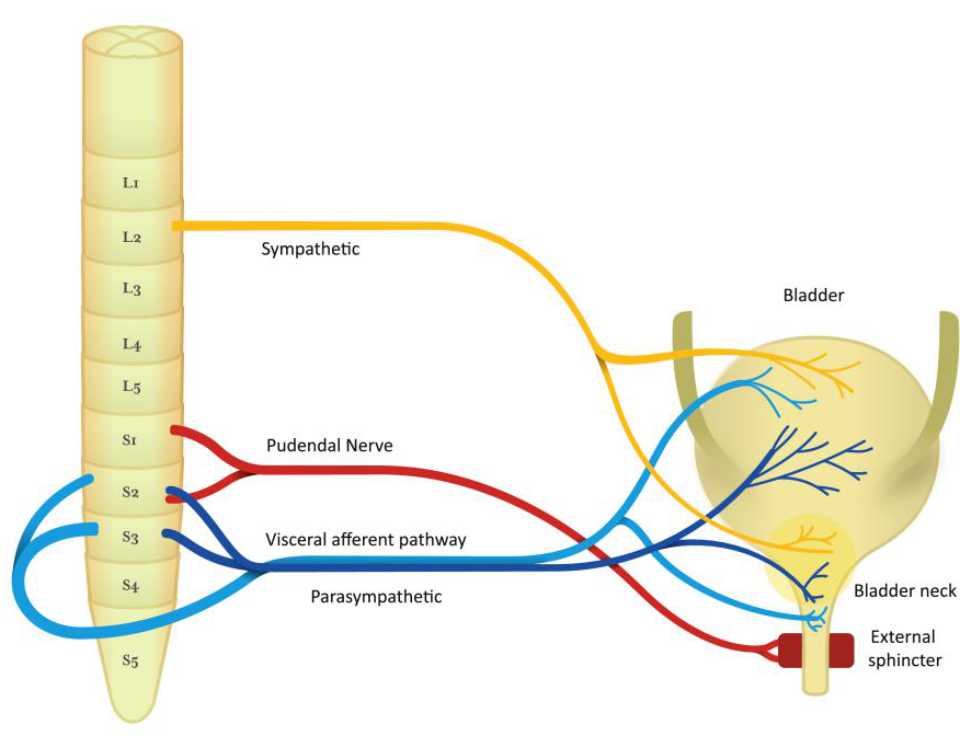
Figure 1. Nerve pathway of bladder function
The detrusor muscle contraction in sacral micturition reflex pathway is started by excitation of bladder afferent and followed by bladder efferent. The balance of voiding control is influenced by pontine micturition center. Therefore, involuntary voiding can occur as a result of any signaling problem in central inhibition or sensitization of bladder afferent.7,10
In bladder storage process, there are two reflexes: guarding reflex and bladder afferent loop reflex. The guarding reflex prevents urine loss from any stress that turned off by supra-pontine input to control bladder emptying. The bladder afferent reflex, responsible for promoting continence during bladder filling, is mediated by pudendal nerve efferent pathways, activated by sacral interneuron, that directly control urethral sphincter.
In bladder emptying process, stimulation of bladder afferent nerve fibers (Aδ or C fibers) comes from any stimulus, like pain, pressure, stretch, and fullness. Then, these fibers connect with parasympathetic efferent regulating bladderbladder reflex and parasympathetic efferent regulating bladder-urethral reflex. As a result, bladder-urethral reflex is inhibited and bladderbladder reflex is permitted leading to voiding.10
It is presumed that in the spinal cord, there are reflexes induced by nerve stimulation. Based on the theory, pelvic muscle contractions or detrusor contractions can be influenced by neurostimulation. Sacral nerve stimulation (SNS) is a method using implantable nerve stimulators which is considered invasive. Recently, there are many studies referring to percutaneous tibial nerve stimulation (PTNS) with its less invasive approach. It will be offered when other conservative measures fail.6
Pathophysiology of OAB
In micturition, there are involvement of the central nervous system, the peripheral somatic, autonomic, sensory afferent innervation, and anatomical components of bladder and urethra. Therefore, any malfunction of these components can induce OAB symptoms.8 The typical objective urodynamic finding in OAB is detrusor overactivity (DO). During filling phase, common signs of this unstable bladders are sudden increase of pressure in bladder at low volume and myogenic activity, as well as changes of smooth muscle structure and stimuli responsiveness.7,11
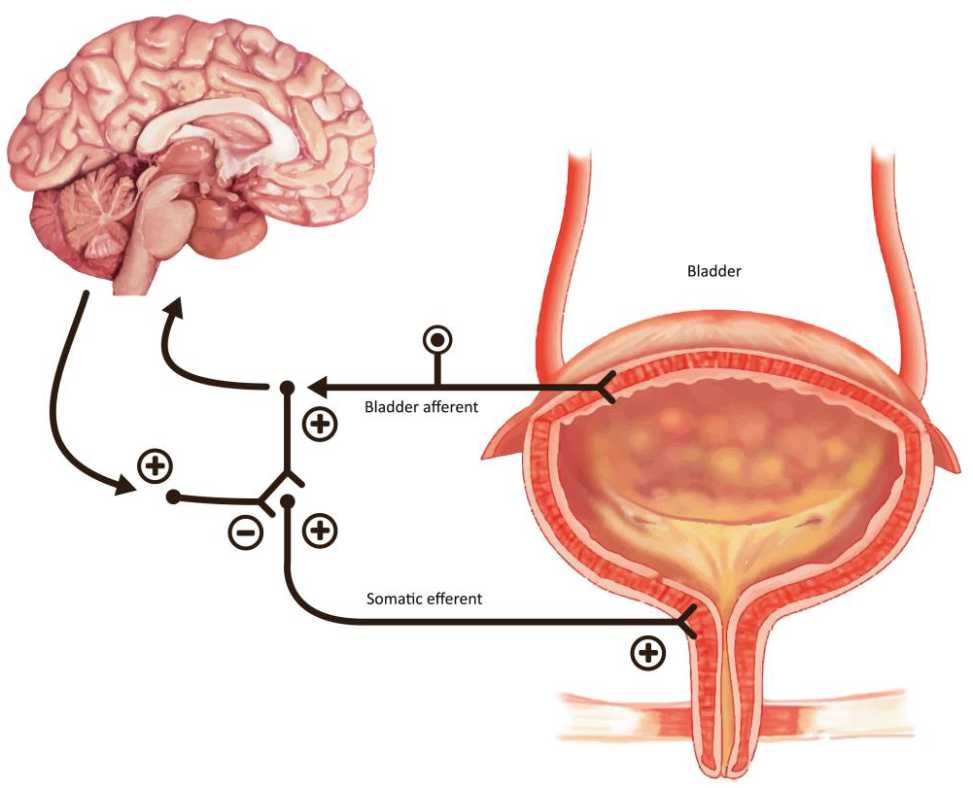
Figure 2. The guarding reflex promotes continence and allows the outlet to contract the urinary sphincter during periods of stress. The brain can turn this reflex off during voiding
Basically, there are three hypotheses that can explain the above findings; neurogenic, myogenic and afferent hypothesis. In the neurogenic hypothesis, the nerve-mediated detrusor muscle excitation induces DO. This excitation may be due to any disturbance in cerebral inhibitory centers, cerebral infarction inducing alteration in several neurotransmitters that are important in micturition, and activation of spinal cord capsaicinsensitive c-fiber-mediated micturition reflex in spinal cord damage above sacral level. In myogenic hypothesis, DO results from a combination of changes in structure and function of bladder detrusor smooth muscle. Therefore, there will be an increase of muscle excitability and enhancement of muscle connectivity generating uninhibited contraction. In afferent mechanism, there are numerous abnormal sensitized afferent nerve endings leading to inappropriately high sensory activity for any distention level of the bladder. In conclusion, the key points in OAB are the degree of excitation and generation in myovesical plexus structures in the bladder wall.7–9
Management
According to The Fourth International Consultation of Incontinence (ICI) recommendations, first line treatment of OAB should be a combination of conservative treatment and pharmacological therapy. In case this method is not successful, it is necessary to perform a more specialized treatment.12
There is a wide range of OAB treatments. To simplify, the management of OAB can be divided into four classes: conservative treatment, pharmacotherapy, surgical therapy, and additional therapy for intractable OAB.7 First option of OAB treatment is conservative or standard treatment including lifestyle modification, pelvic floor muscle exercise and bladder training.6 For lifestyle interventions, patients should pay attention to their dietary irritants (caffeine, alcohol), fluid intake and smoking habit. Exercise of pelvic floor muscle and bladder training will assist patient to resist and terminate overactivity, as well as improving inhibitory mechanism on lower urinary tract.7 The second class treatment is pharmacotherapy. The primary mechanism of action of anti-muscarinic is to decrease urgency and improve bladder capacity. Systematic review and meta-analysis by Chapple et al13 has shown that these medications were better than placebo significantly in incontinence and voiding symptoms. Unfortunately, despite its efficacy and effectiveness, approximately 80% of OAB patients stop antimuscarinic after a year, 17% of them is related to adverse side effects.6,14 The most common adverse events reported are dry mouth and constipation, followed by drowsiness, blurred vision, and declined cognitive function. Patients with such condition have the third line treatment options such as bladder augmentation, detrusor myomectomy, or less invasive methods of treatment like botulinum toxin injection to detrusor muscle and electrical stimulation (neuromodulation and neurostimulation).6,15,16 For intractable OAB, there is a place for appliances, catheters, urethral closure, and urinary diversion.7
Most OAB patients can take advantages from multimodal therapy consisting of medical therapy, bladder training and pelvic floor muscle training (PFMT). When symptoms do not disappear at the maximal dosage of pharmacotherapy, called refractory OAB, the remaining options are neuromodulation or surgery.7
Electrical stimulation
In 1878, the early finding of direct bladder stimulation via a metal transurethral catheter in urinary retention patients increased development of bladder direct stimulation through detrusor and transurethral routes. On the other hand, the direct stimulation of pelvic nerve in 1965 seemed to be unsuitable for treatment of bladder dysfunction. It was due to increase of outlet resistance through stimulation of hypogastric nerve. Therefore, stimulation methods were more focused on the spinal cord, sacral roots, or pelvic floor muscles.10
In 1990, the studies showed that stimulation of sacral anterior root accompanied with posterior sacral 2 (S2) to sacral 4 (S4) rhizotomies was necessary for optimal bladder emptying as it would increase bladder compliance and decrease detrusor activity.
Then, the concept of neuromodulation was defined as generation of external sphincter function by sacral roots activation that decrease normal inhibitory reflex of detrusor. These findings made the basic knowledge of neurostimulation and neuromodulation.10
Neurostimulation is the electrical stimulation applied on nerves and muscles to get rapid clinical changes in neurogenic conditions, while neuromodulation is the electrical stimulation of nerves to make neurotransmission process alteration, both in neurogenic and non-neurogenic condition.10
In order to find methods of restoring complete voluntary control over storage and emptying function of the bladder, many researches have been performed. There are several ways of electrical stimulation which are determined by the purpose or selection of the desired bladder function and sites of stimulation with the related mechanism (Table 1).17
Table 1. Choices of electrical stimulation in voiding dysfunction treatment10
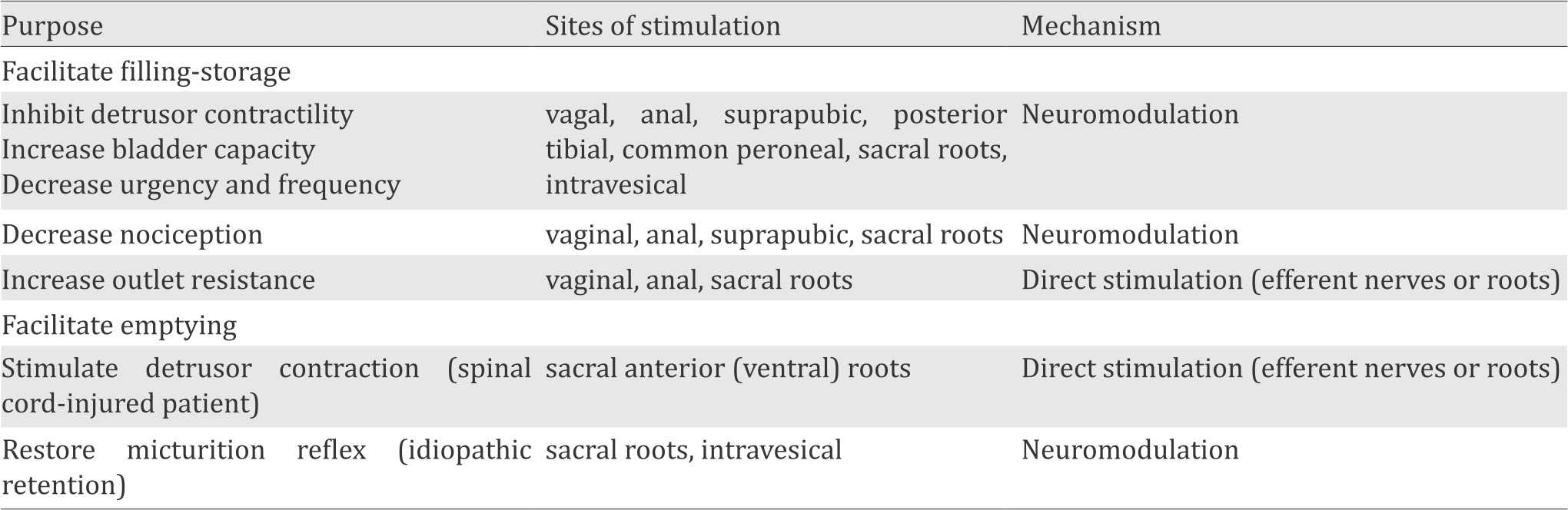
In patients with detrusor hyperreflexia, neurostimulation of sacral roots showed effective hyperactivity suppression. This suppression of detrusor contractility is a result of the sacral plexus somatic component stimulation by reflex response of detrusor muscle.10
Sacral nerve stimulation (SNS) has been proved to be effective in the treatment OAB but is considered invasive and related to complications such as infection, pain, and need of surgical revision because of lead migration in 33% of cases. In SNS procedure, implanted device is used to stimulate S3 and nearby nerve roots.18 Other stimulation called transcutaneous electrical nerve stimulation (TENS) has been explained using electrodes in supra-pubic or sacral attachment. In spite of its high continence rates (54%), the long therapy is associated with skin irritation.19 Gungor Ugurlucan et al20 assessed the comparison of percutaneous tibial nerve stimulation (PTNS) and transvaginal electrical stimulation (ES) in OAB treatment. The result showed that both ES and PTNS were effective in OAB treatment with significant improvement in subjective and objective parameters, but without significant difference between the two groups.20 Thus, PTNS is considered more effective than TENS and suggested as a cheaper, safer and less invasive alternative to SNS and ES.21
Percutaneous tibial nerve stimulation (PTNS) Definition
PTNS is the least invasive form of neuromodulation that percutaneously stimulate posterior tibial nerve and deliver electrical stimulation to sacral nerve plexus. This plexus consists of sensory and motor nerve fibers originating from lumbal 4 (L4) through sacral 3 (S3) which control the function of bladder, urinary sphincter, and pelvic floor muscles, both through the somatic and autonomic nerves. The system may have an effect both on the detrusor and micturition centers in the brain.10,22 PTNS pathway to sacral plexus is represented in Figure 3.
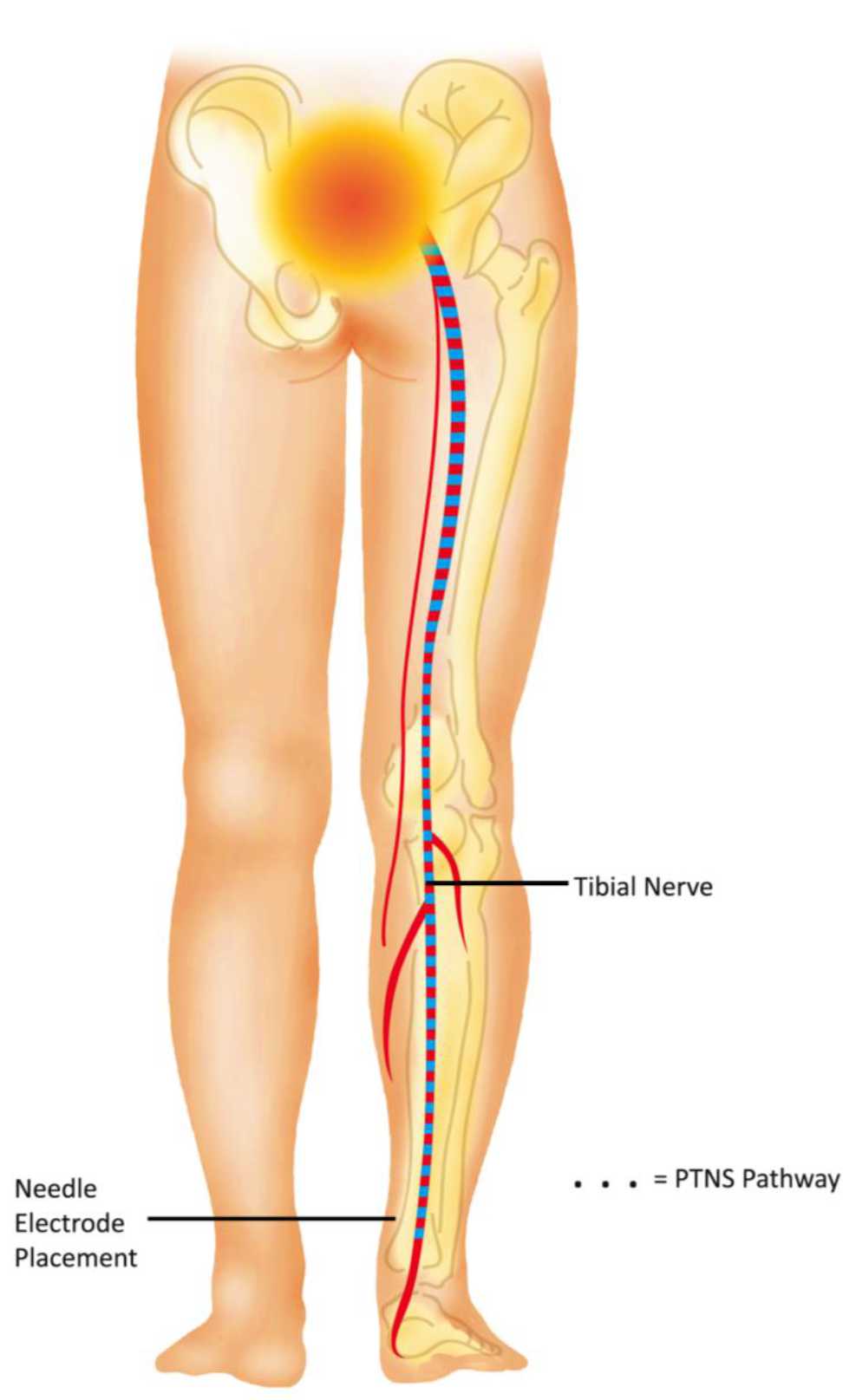
Figure 3. PTNS pathway to sacral plexus
PTNS (Urgent PC, Anoka, Mn, CystoMedix) is approved by U.S. FDA. It was first improved and promoted by Stoller.1 The use of PTNS for refractory OAB is recommended by European Association of Urology (EAU), American Urological Association (AUA), and National Institute for Health and Clinical Excellence (NICE).23–25
Procedure
There is no special preparation for doing PTNS. The patients are asked to sit comfortably in a chair with a leg elevated. A small, slim needle, specifically 34-gauge needle, is inserted percutaneously near the ankle, just posterior to tibia margin and 5 cm cephalad from the medial malleolus. On the medial surface of the calcaneus, the grounding electrode pad is placed. An adhesive pad will be applied to the foot to complete the circuit. It is important to insert the needle at the right location. These settings are illustrated in Figures 4 and 5.
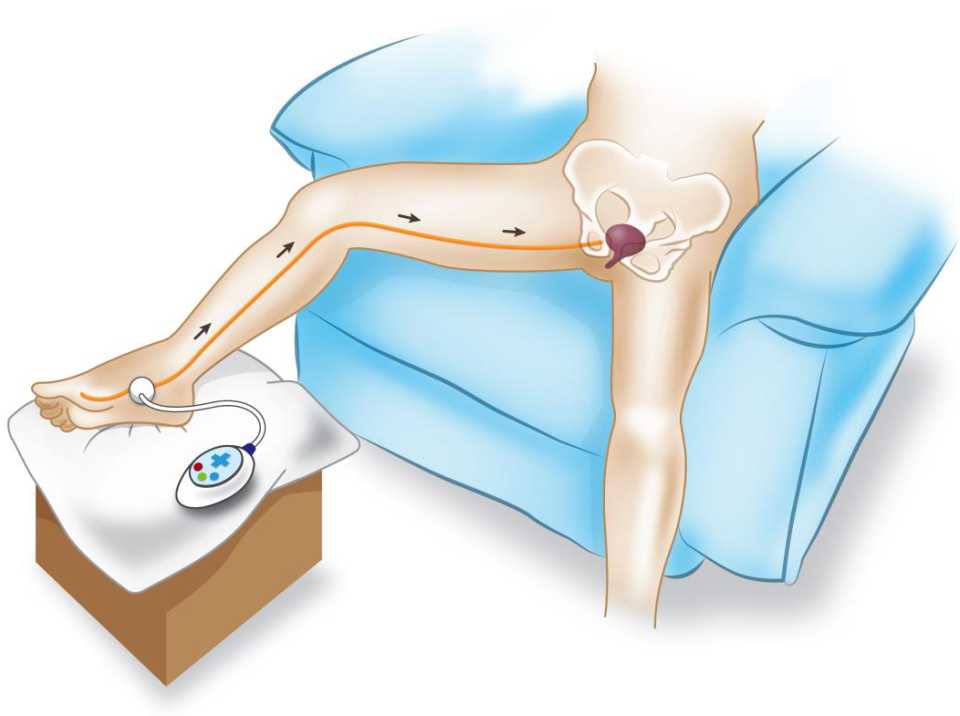
Figure 4. Setting of PTNS procedure
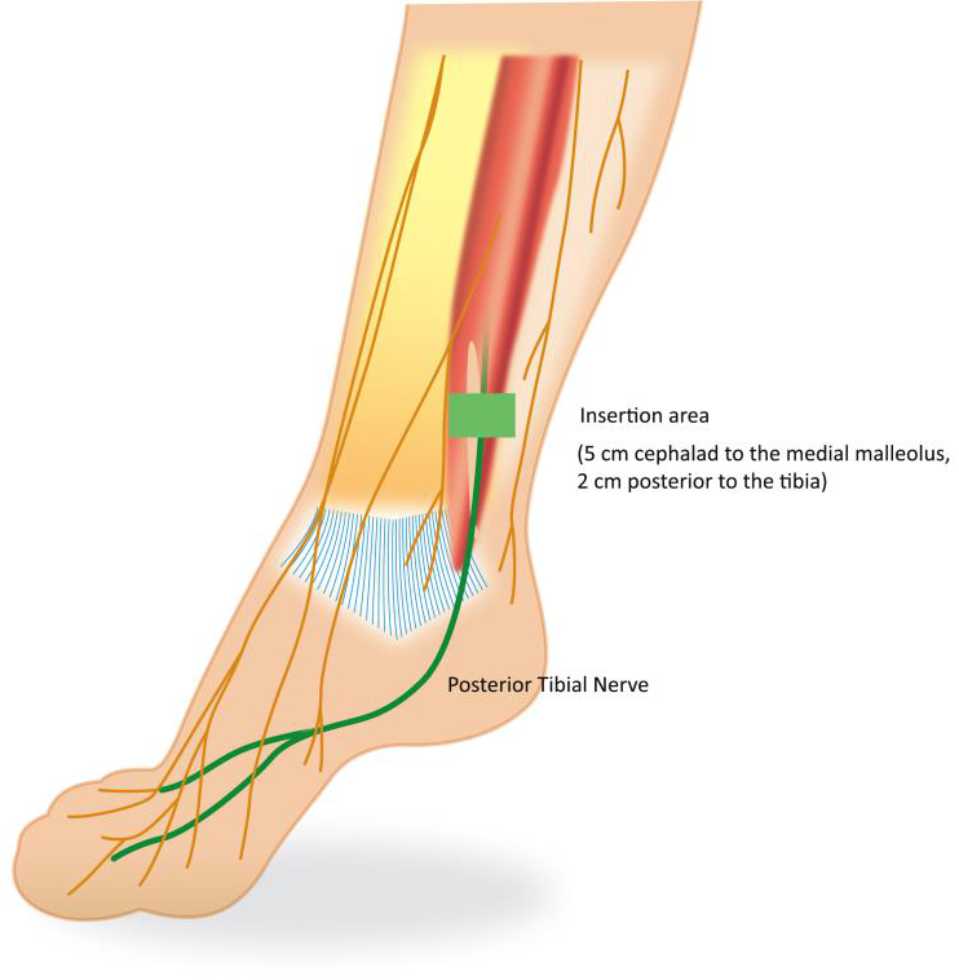
Figure 5. Needle electrode placement
The component of PTNS is connected to a batterypowered stimulator and then stimulator is turned on. It is necessary to adjust the strength of stimulation. First, the sensation will emerge in the ankle, foot, toes and spread out. The patient’s response should be observed to get the ideal strength of impulse needed. Then, stimulator’s impulses are delivered to tibial nerve, transferred to sacral nerve plexus and thereby stimulate the inhibition of detrusor contractility.10
Commonly, PTNS cycles consist of 12-weekly treatment of 30 minutes with parameters evaluated are nocturia and urge incontinence after four to six treatment. At least six treatments of PTNS are needed for the patients to get a change of symptoms. Patients who successfully respond to PTNS treatment need retreatment to get sustainable results.10,24,26
Contraindication
The contraindications of PTNS are patients with pacemaker, nerve damage, coagulopathy that favors bleeding, and pregnancy.
Efficacy
The early multicenter trial by Govier et al21 found that from 53 patients with a various lower urinary tract diseases, there were 71% response after 12 weekly PTNS treatments. The responders with positive response of PTNS were also shown in several studies from 2005 to 2006, ranging from 37.3% to 81.8%.27–31 The terms of responders were more than 50% reduction in symptoms including urgency, frequency, incontinence episodes,27,29,30 complete recovery after treatment,28 overall treatment response rate31 and subjective feeling of improvement.27 In an RCT by Finazzi Agrò et al32 comparing PTNS and sham procedure, there were 75% and 0% patients with 50% reduction in urinary incontinence episodes in PTNS and sham group, respectively.
After its approval by FDA in 2007, PTNS showed considerable promise in the management of OAB. There were several non-comparative and comparative studies comparing PTNS with sham procedure, anti-muscarinic therapy, and multimodal therapy consisting of PTNS and antimuscarinic for the treatment of OAB.
In non-comparative studies, Burton et al19 found in their meta-analysis that the PTNS pooled objective success rate was 60.6% (95% CI=49.2–74.7) and subjective success rate was 61.4% (95% CI=57.5–71.8).
Efficacy of PTNS in OAB has also been proven in sham-controlled trials. When compared to sham procedure, the number or percentage of responders in PTNS group were statistically higher (p<0.01), not only in objective responses32-34 but also in global response assessment (GRA) improvement or cure.35 The significant reduction of symptoms in PTNS group compared to sham group were found in frequency, urinary incontinence, and nocturia episodes.32,33,35 In the matter of voided volume, two studies comparing PTNS and sham procedure by Agro et al33 and Agro et al35 showed significant increase of voided volume in PTNS group (p≤0.001) but not significant in sham group. The efficacy of PTNS in OAB is supported in meta-analysis by Burton et al19 which concluded that patients who received PTNS treatment have seven times possibility to get improvement compared to sham treatment (RR=7.02 [95% CI=1.69–29.17]). The same result was shown in another meta-analysis by Wibisono36 with overall risk ratio of 7.32 (95% CI=1.69–32.16) favouring PTNS over sham procedure and with number needed to treat 4.28 (95% CI=3.19–6.49).
In studies comparing PTNS and anti-muscarinic, there were variable results in changes of voiding diaries symptoms. Most of them showed significant reduction of symptoms in both groups with no significant difference.37–39 Peters et al38, with study subjects of 100 OAB patients, compared PTNS with tolterodine 4 mg/day with similar results in voiding diaries symptom reduction. However, symptoms improvement was reported by more subjects treated with PTNS (79.5%) than tolterodine (54.8%) (p=0.010).
In comparison of PTNS and multimodal therapy, there was significant difference of symptoms episodes favouring multimodal therapy in a study by Sancaktar et al40 and significantly higher response rate of multimodal therapy group compared to PTNS reported by Karademir et al.31 In the treatment of neurogenic OAB specifically, PTNS showed similar results. Kabay et al41 evaluated 32 patients with Parkinson’s disease accompanied with neurogenic detrusor DO. They found significant improvement of urodynamic parameters in patients treated with PTNS including mean first involuntary detrusor (first IDCV) contraction and mean maximum cystometric capacity (MCC) after PTNS.41 It was supported by the study of PTNS in multiple sclerosis patients with OAB symptoms where there were significant reduction of daytime frequency, nocturia, and mean post-micturition residual, as well as increase of mean voided volume. Approximately, 89% patients had a treatment satisfaction of 70%.42
In children, PTNS is also proved feasible, safe and minimally painful. De Gennaro et al43 reported that 80% of children with OAB had improved their symptoms which were 44% totally dry. Children had significant improvement in bladder capacity and urinary retention symptoms as much as 62.5% and 71%, respectively. The faces rating scale results introduce no severe pain face with concurrent visual analog scale results showing a low score with a further decrease during the first, sixth, and twelfth sessions (p<0.05).43 In another study, Hoebeke et al44 prospectively demonstrated similar success with 32 therapy-resistant children, non-neurogenic bladder sphincter dysfunction. Out of the 28 children with pretreatment urgency, seven children experienced disappearance of their urgency and 10 children had improvement. After stimulation, four out of 23 children with daytime incontinence became dry, 12 of them had their incontinence decreased. A daily normal one of four to six voids achieved in 16 out of 19 children with abnormal voiding frequency.10,44
Long-term efficacy
From the information above, the short-term therapy of PTNS should be beneficial in the treatment of OAB. However, symptoms deteriorate within 6–12 weeks without ongoing treatment. Thus, it will be necessary to explore more information on long-term therapy.19 Macdiarmid et al45 evaluated the durability in long-term of PTNS in OAB by the continuation of the second phase of the overactive bladder innovative therapy trial (OrBIT) where 33 responders of the PTNS group received an additional nine months of PTNS treatment. There was significant (statistically) improvement of OAB symptoms with 12 weekly PTNS that demonstrated good durability of 12 months through. This conclusion was obtained from 12 months mean improvements from the baseline in frequency, urge incontinence, nocturia, and voided.45 In addition, there was sustained therapeutic effects of PTNS (STEP) study which was the continuation from Study of Urgent PC versus Sham Effectiveness in Treatment of Overactive Bladder Symptoms (SuMIT) evaluating long-term efficacy of PTNS. After successful 12 weekly treatments, patients continued with 14-week tapering protocol and personalized treatment plan. Frequency, urge incontinence, nocturia, and urgency episodes improvements were statistically significant compared to baseline at six, 12, 18, and, 24 months.46 At three years, they found 77% (95% CI=64%–90%) of subjects with maintained or marked OAB improvements.47 All in all, PTNS is durable and can be a long-term treatment option for OAB.
Adverse events
The side effects of PTNS are not frequent and considered minimal. They include minor irritation (redness or inflammation), ankle bruising, discomfort or pain near the stimulation site or around ankle, tingling in leg, generalized swelling, toe numbness, stomachache, headache, hematuria, bleeding where needle has been inserted, and inability to tolerate stimulation.19,29,35,38 They were found in a few number of patients and considered rare. In long-term PTNS therapy (STEP study), the events reported were slow stream, UTI, bladder pressure, pulling feeling on feet, and pinched nerve, with no direct relationship to PTNS.46 In patients receiving anti-muscarinic, the common adverse events were constipation, infection, dizziness, visual disturbance, and fatigue.38
In a study comparing PTNS and anti-muscarinic, a well toleration with negligible serious adverse events reported in both treatments. It was stated that after 12 weeks of therapy, several symptoms were reported significantly less in the PTNS group compared to the anti-muscarinic group, including dry mouth and constipation.38 A study of Peter et al35 showed no serious adverse events reported in sham group and PTNS group.
In conclusion, many of millions people suffering from OAB do not respond well to the medical and behavioral therapy. PTNS offers these refractory patients a safe, effective and minimally invasive treatment.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
REFERENCES
- Payne CK. Conservative Management of Urinary Incontinence: Behavioral and Pelvic Floor Therapy, Urethral and Pelvic Devices. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 1. 10th ed. Philadelphia: Elsevier Saunders; 2012. p. 2026–46.
- Staskin D, Kelleher C, Bosch R, Cotterill N, Coyne K, Kopp Z, et al. Initial assessment of urinary incontinence in adult male and female patients. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 5th International Consultation on Incontinence. France: ICUD-EAU; 2013. p. 361–88.
- Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, Sand P, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourology and urodynamics. 2009;28(4):287.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology and urodynamics. 2002;21(2):167–78.
- Sumardi R, Mochtar CA, Junizaf, Santoso BI, Setiati S, Nuhonni SA, et al. Prevalence of urinary incontinence, risk factors and its impact: multivariate analysis from indonesian nationwide survey. Acta medica Indonesiana. 2014;46(3):175–82.
- Percutaneous tibial nerve stimulation for the treatment of overactive bladder [Internet]. California Technology Assessment Forum. 2012 [cited June 20, 2012]. Available from: http://ctaf.org/sites/default/files/assessments/ PTNS June 2012_0.pdf.
- Drake M, Abrams P. Overactive bladder. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell- Walsh Urology. 1. 10th ed. Philadelphia: Elsevier Saunders; 2012. p. 1947–56.
- Banakhar MA, Al-Shaiji TF, Hassouna MM. Pathophysiology of overactive bladder. International urogynecology journal. 2012;23(8):975–82.
- Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002;4(Suppl 4):S7–18.
- Vasavada SP, Rackley RR. Electrical stimulation and neuromodulation in storage and emptying failure. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 1. 10th ed. Philadelphia: Elsevier Saunders; 2012. p. 2026–45.
- Chu FM, Dmochowski R. Pathophysiology of overactive bladder. The American journal of medicine. 2006;119(3):3–8.
- Abrams P, Andersson K, Birder L, Brubaker L, Cardozo L, Chapple C, et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourology and urodynamics. 2010;29(1):213–40.
- Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects of antimuscarinic treatments in overactive bladder: a systematic review and meta-analysis. European urology. 2005;48(1):5–26.
- Gopal M, Haynes K, Bellamy SL, Arya LA. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstetrics & Gynecology. 2008;112(6):1311–8.
- Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Cardozo L, Subramanian D, et al. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. International journal of clinical practice. 2011;65(5):567–85.
- Brostrom S, Hallas J. Persistence of antimuscarinic drug use. European journal of clinical pharmacology. 2009;65(3):309–14.
- Tanagho EA, Bella AJ, Lue TF. Neuropathic bladder disorders. In: Tanagho EA, McAninch JW, editors. Smith’s general urology. 17th ed. New York: McGraw- Hill Medical; 2008. p. 438–54.
- Herbison GP, Arnold EP. Sacral neuromodulation with implanted devices for urinary storage and voiding dysfunction in adults. The Cochrane Library. 2009.
- Burton C, Sajja A, Latthe P. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: A systematic review and meta‐analysis. Neurourology and urodynamics. 2012;31(8):1206–16.
- Gungor Ugurlucan F, Onal M, Aslan E, Ayyildiz Erkan H, Kizilkaya Beji N, Yalcin O. Comparison of the effects of electrical stimulation and posterior tibial nerve stimulation in the treatment of overactive bladder syndrome. Gynecologic and obstetric investigation. 2013;75(1):46–52.
- Govier FE, Litwiller S, Nitti V, Kreder KJ, Rosenblatt P. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. The Journal of urology. 2001;165(4):1193–8.
- van der Pal F, Heesakkers JP, Bemelmans BL. Current opinion on the working mechanisms of neuromodulation in the treatment of lower urinary tract dysfunction. Current opinion in urology. 2006;16(4):261–7.
- Chen Y-F, Bramley G, Unwin G, Dretzke J, Moore D, Hanu- Cernat D. Systematic Reviews referred by the NICE Interventional Procedures Programme on behalf of the NICE Interventional Procedures Advisory Committee (IPAC) Title Stimulation of peripheral nerves for the treatment of refractory pain (including peripheral nerve field. 2012.
- Lucas M, Bedretdinova D, Bosch J, Burkhard F, Cruz F, Nambiar A, et al. EAU guidelines of urinary incontinence. 2013.
- Gormley EA, Lightner DJ, Burgio KL, Chai TC, Clemens JQ, Culkin DJ, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. The Journal of urology. 2012;188(6):2455–63.
- MacDiarmid SA, Staskin DR. Percutaneous tibial nerve stimulation (PTNS): a literature-based assessment. Current Bladder Dysfunction Reports. 2009;4(1):29–33.
- Van Balken M, Vergunst H, Bemelmans B. Prognostic factors for successful percutaneous tibial nerve stimulation. European urology. 2006;49(2):360–5.
- Nuhoğlu B, Fidan V, Ayyıldız A, Ersoy E, Germiyanoğlu C. Stoller afferent nerve stimulation in woman with therapy resistant over active bladder; a 1-year follow up. International urogynecology journal. 2006;17(3):204–7.
- Van Der Pal F, Van Balken MR, Heesakkers JP, Debruyne FM, Bemelmans BL. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU international. 2006;97(3):547–50.
- Finazzi Agro E, Campagna A, Sciobica F, Petta F, Germani S, Zuccala A, et al. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2005;57(2):119–23.
- Karademir K, Baykal K, Sen B, Senkul T, Iseri C, Erden D. A peripheric neuromodulation technique for curing detrusor overactivity: Stoller afferent neurostimulation. Scandinavian journal of urology and nephrology. 2005;39(3):230–3.
- Finazzi Agro E, al. e. Percutaneous tibial nerve stimulation (PTNS) in the treatment of urge incontinence: A double blind placebo controlled study. SIUD National Congress; Italy: Uroplasty; 2005.
- Finazzi-Agrò E, Petta F, Sciobica F, Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, doubleblind, placebo controlled trial. The Journal of urology. 2010;184(5):2001–6.
- Finazzi‐Agrò E, Rocchi C, Pachatz C, Petta F, Spera E, Mori F, et al. Percutaneous tibial nerve stimulation produces effects on brain activity: study on the modifications of the long latency somatosensory evoked potentials. Neurourology and urodynamics. 2009;28(4):320–4.
- Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. The Journal of urology. 2010;183(4):1438–43.
- Wibisono E, Rahardjo HE. Effectiveness of short term percutaneous tibial nerve stimulation for nonneurogenic overactive bladder syndrome in adults: a systematic review and meta analysis. Acta Med Indones. 2015;47(3):188–200.
- Souto SC, Reis LO, Palma T, Palma P, Denardi F. Prospective and randomized comparison of electrical stimulation of the posterior tibial nerve versus oxybutynin versus their combination for treatment of women with overactive bladder syndrome. World journal of urology. 2014;32(1):179–84.
- Peters KM, MacDiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, et al. Randomized trial of percutaneous tibial nerve stimulation versus extendedrelease tolterodine: results from the overactive bladder innovative therapy trial. The Journal of urology. 2009;182(3):1055–61.
- Vecchioli-Scaldazza C, Morosetti C, Berouz A, Giannubilo W, Ferrara V. Solifenacin succinate versus percutaneous tibial nerve stimulation in women with overactive bladder syndrome: results of a randomized controlled crossover study. Gynecologic and obstetric investigation. 2012;75(4):230–4.
- Sancaktar M, Ceyhan ST, Akyol I, Muhcu M, Alanbay I, Mutlu Ercan C, et al. The outcome of adding peripheral neuromodulation (stoller afferent neuro-stimulation) to anti-muscarinic therapy in women with severe overactive bladder. Gynecological Endocrinology. 2010;26(10):729–32.
- Kabay SC, Kabay S, Yucel M, Ozden H. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourology and urodynamics. 2009;28(1):62–7.
- Gobbi C, Digesu G, Khullar V, El Neil S, Caccia G, Zecca C. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Multiple Sclerosis Journal. 2011;17(12):1514–9.
- De Gennaro M, Capitanucci M, Mastracci P, Silveri M, Gatti C, Mosiello G. Percutaneous tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunction. The Journal of urology. 2004;171(5):1911–3.
- Hoebeke P, Renson C, Petillon L, Walle JV, De Paepe H. Percutaneous electrical nerve stimulation in children with therapy resistant nonneuropathic bladder sphincter dysfunction: a pilot study. The Journal of urology. 2002;168(6):2605–8.
- MacDiarmid SA, Peters KM, Shobeiri SA, Wooldridge LS, Rovner ES, Leong FC, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. The Journal of urology. 2010;183(1):234–40.
- Peters KM, Carrico DJ, MacDiarmid SA, Wooldridge LS, Khan AU, McCoy CE, et al. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24‐ month results of the STEP study. Neurourology and urodynamics. 2013;32(1):24–9.
- Peters K, Carrico D, editors. Percutaneous tibial nerve stimulation for the treatment of overactive bladder syndrome: final 36-month results of the STEP study. Neurology and Urodynamics; 2013: Wiley-Blackwell.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id
