
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Increased vimentin mRNA expression in MCF-7 breast cancer cell line after repeated endoxifen-treatment
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i4.1397 Med J Indones. 2016;25:207–13
Received: March 01, 2016
Accepted: December 19, 2016
Author affiliation:
1 Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Departement of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Melva Louisa
E-mail: melva.louisa@gmail.com
Background
Epithelial mesenchymal transition (EMT) plays a significant role in the development of cancer cell resistance to drugs. Vimentin, a type III intermediate filament protein, is a marker of EMT. Vimentin's overexpression in cancer correlates well with increased tumor growth, change in cell shape and poor prognosis. Endoxifen is an active metabolite of tamoxifen and has become a new potent agent in the treatment of breast cancer. This is a study that aimed to investigate the effect of endoxifen exposure with or without estradiol on cell viability, cell morphology and EMT progression through the analysis of vimentin mRNA expression after 4-week treatment.
Methods
Endoxifen, 100 nM or 1,000 nM, with or without beta-estradiol were given repeatedly to MCF-7 cells. Cells treated with dimethyl sulfoxide (DMSO) 0.001% were used as control. After 2- and 4-week exposure, the cells were counted, analyzed for mRNA vimentin expression, and observed for morphological changes.
Results
Compared to control, there were significant decreases in vimentin mRNA expressions in endoxifen and endoxifen+β-estradiol treated cells after 2-weeks, which then significantly increased after 4-week compared with the 2-week exposure. We found no change in morphology of MCF-7 cells.
Conclusion
Repeated exposure of endoxifen might induce EMT progression through increased expression of vimentin in MCF-7 breast cancer cell line.
Keywords
endoxifen, EMT, vimentin
Tamoxifen, a prodrug, is commonly given to women diagnosed with estrogen receptor (ER)- positive breast cancer. In human body, tamoxifen is metabolized into 4-hydroxy tamoxifen (4HT) and N-desmethyl-tamoxifen (NDT) by the cytochrome P-450 enzyme system, followed by secondary metabolism to 4-hydroxy-N-desmethyl-tamoxifen (endoxifen).1,2
4HT is assumed as the primary means by which tamoxifen exerts its anti-breast cancer effect. However, current studies suggest that endoxifen is equipotent to 4HT and significantly more potent than tamoxifen in its capability to bind to estrogen receptor a and b (ERa and ERb), and also in suppressing ER-dependent breast cancer proliferation.1
Currently, endoxifen is being developed as a novel drug for the treatment of endocrine responsive breast cancer patients.3 Hawse et al2 showed that endoxifen had similar actions to selective estrogen receptor antagonist (fulvestrant) with regard to its ability to degrade ERα through proteasome degradation system, to block ERα-mediated transcriptional activation and to prevent estrogeninduced breast cancer cell proliferation.2 A study was conducted by Wu et al1 and showed that cells treated with high endoxifen (E) concentrations, i.e. 100–1,000 nmol/L resulted in significant decreases in ERα protein levels. Otherwise, low endoxifen concentrations (20-40 nmol/L) did not induce degradation of ERα protein.
Cancer cell resistance to drugs is one of major issues that developed in the use of therapeutic drugs for breast cancer treatment. There are some categories of mechanisms that promote direct or indirect drug resistance in human cancer cells such as inactivation of drug, alteration of drug target, drug efflux, deoxyribonucleic acid (DNA) damage repair, inhibition of cell death and epithelial-mesenchymal transition (EMT).4 A recent study showed that exposure of endoxifen 1,000 nmol/L for 15 months induced epithelialmesenchymal transition (EMT) in Michigan Cancer Foundation (MCF-7) cells.3
Epithelial-mesenchymal transition is a cellular reprogramming process in which cells change their epithelial towards mesenchymal phenotype that lead the cells to alter their shape and show increased motility. During EMT, there are reorganization of cytoskeletal elements including replacement of peripheral actin cytoskeleton and cytokeratin intermediate filaments with stress fibers and vimentin, respectively. Because of this dramatic changes in intermediate filament (IF) composition, vimentin expression has become a canonical marker of the EMT.5–11
Vimentin, a type III IF protein, is found in normal mesenchymal cells and is known to maintain integrity of cells and provide resistance against stress.5 Mendez et al6 showed that during epithelial to mesenchymal transition process, vimentin can cause alteration in cell shape, motility, and adhesion.
The positive correlation between increased migration and invasion of cancer cells and elevated vimentin expression has been found.12,13 In the MCF-7 cells, vimentin gene transfection caused increased invasiveness.13 A previous study demonstrated that invasive breast cancer cell lines expressed more vimentin, suggesting its important role in identifying cases with worse prognosis.12
Our study was aimed to investigate the effect of repeated exposure of endoxifen with or without β-estradiol to MCF-7 breast cancer cell line on cell viability, morphology and EMT progression through the analysis of vimentin mRNA expression.
METHODS
Study design
This was an in vitro experimental study in a commercially available breast carcinoma cell line, MCF-7; therefore ethical clearance is exempted. This study was conducted in Pharmacokinetics Laboratory, Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, from March to September 2015.
Cell culture
MCF-7 human breast carcinoma cell line was kindly provided by Makmal Terpadu Laboratory, Faculty of Medicine, Universitas Indonesia. MCF-7 cell morphology was characterized by cobblestone-like appearance and strong cell–cell adhesion.14 The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM)-high glucose supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/mL streptomycin, and 2.5 μg/ml fungizone. Cells were counted, then 10,000 cells were seeded into triplicate wells of 6-well plates and growth was assessed at about 90% confluency, following trypsinisation and resuspension in medium, by direct cell counting using a hemocytometer.
Doubling time and growth curve analysis
Cells were harvested at day 1, 2, 3, 4, 5, 6, 7 and 9. Afterwards the cells were counted and plotted in a growth curve. Cell doubling time were calculated using the doubling time calculation provided in http://www.doubling-time.com.
Drug treatment
MCF-7 cells were plated at 10,000 cells and were treated with endoxifen (100 nmol/L and 1,000 nmol/L) with or without b-estradiol 1 nmol/L in 6-well plates. Endoxifen and estradiol were dissolved in dimethyl sulfoxide (DMSO) 0.001%. Therefore, the control used was DMSO 0.001%. Every seven days, the cells were trypsinized, counted and 10,000 cells were replated in complete medium. Drug treatments were continuously given for four weeks, three times per week along with medium changes. Cell viability assays were done every week. At the end of second and fourth week of drug treatment, cells were harvested and isolated for ribonucleic acid (RNA) and further synthesized to complementary DNA (cDNA) for quantitative realtime polymerase chain reaction (PCR) analysis.
Cell viability assay
Cell viability assay was performed by using trypan blue dye exclusion assay. The numbers of viable cells were counted by trypan blue dye exclusion using a hemocytometer. The results were expressed as percentage of viable cells in given drug treatments relative to the viable cells in DMSO group.
Quantitative realtime PCR analysis
Total cellular RNA was extracted from 1,000,000 cells after trypsinized from six well plates using High Pure RNA isolation kit (Roche, USA) according to the manufacturer’s protocol. Further, 1 μg (in 20 μl) was converted to cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche, USA). Quantitative realtime PCR was performed on 100 ng cDNA using a FastStart DNA master SYBR Green I kit (Roche, USA), according to manufacturer’s protocol. As reference, β-actin, a housekeeping gene, was used. Primer sequences used for vimentin: forward primer was 5’-GACAATGCGTCTCTGGCACGTCTT-3’ and reverse primer:was 5’-TCCTCCGCCTCCTGCAGGTTCTT-3’; for β-actin: forward primer was 5’-GCTGGAAGGTGGACAGCGA-3’ and reverse primer was 5’-GGCATCGTGATGGACTCCG-3’.
A total of 20 μl reactions were performed in a 32- well plate on an qRT-PCR Light Cycler Nano RocheTM by incubation at 95°C for 10 minutes, followed by 45 cycles of 95°C for 20 seconds and 65°C for one minute. The raw quantification cycle (Cq) values were analysed by relative quantification using Livak method to determine normalized expression ratios of vimentin to b-actin.
Statistical analysis
Data collected were cell viability, morphology and vimentin expression. Cell morphologies were presented as figures, and MCF-7 cell viabilities and vimentin mRNA expressions were presented as mean ± standard deviation (SD) of three experiments. Statistical analyses were performed with one-way ANOVA. p<0.05 was taken to be statistically significant difference. If one-way ANOVA showed significant difference, then we proceeded with Tukey’s multiple comparison method.
RESULTS
Optimization of cell culture
The growth curve showed that MCF-7 cells had an exponential growth pattern with a doubling time of 24.51 hours (Figure 1).
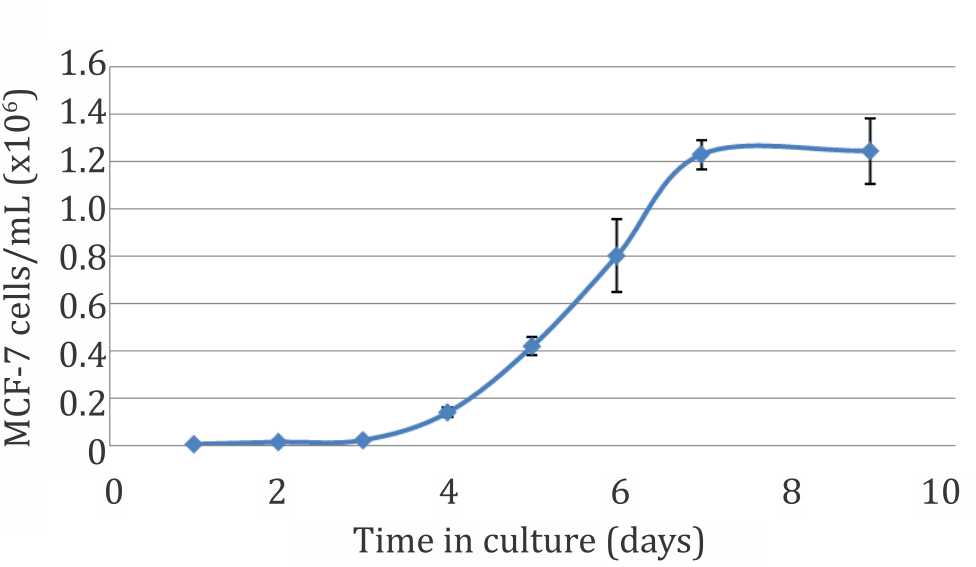
Figure 1. MCF-7 cell growth curve
The effects of endoxifen on MCF-7 cell viability
Viabilities were expressed as percentage relative to the control (cells exposed to DMSO 0.001%). Results are presented as means of three experiments (Figure 2).
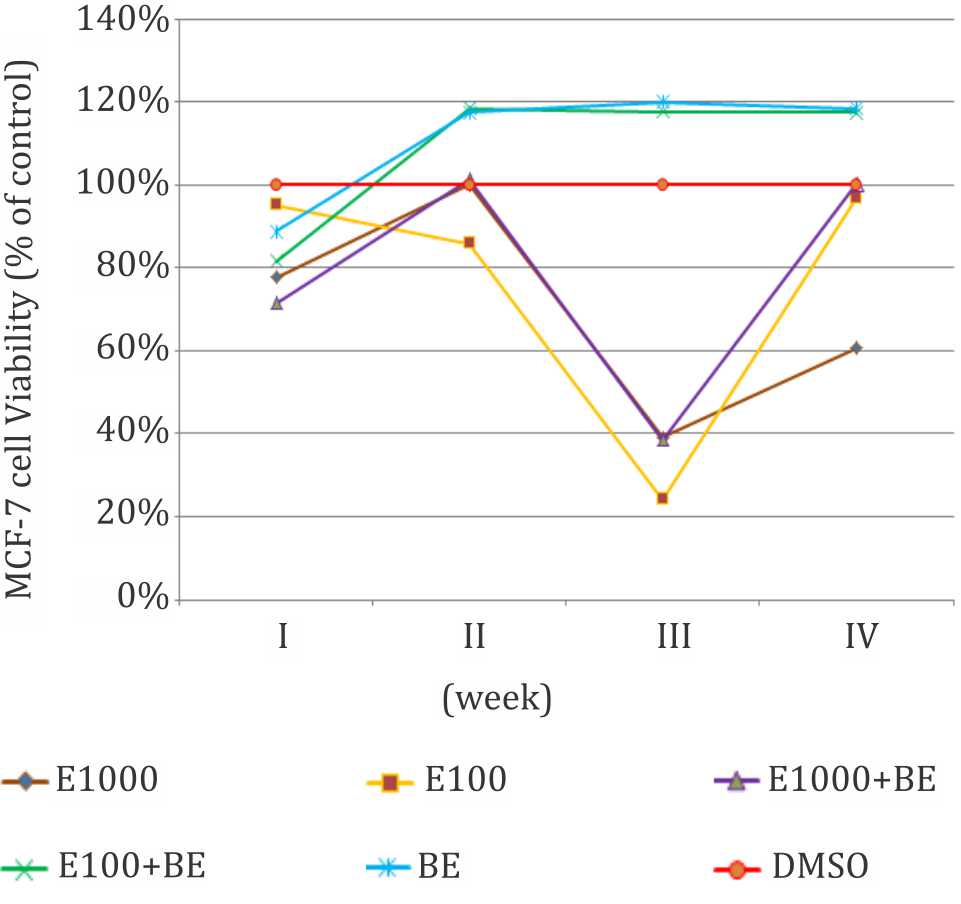
Figure 2. Effect of endoxifen with or without β-estradiol on MCF-7 cells viability. E1000= endoxifen 1,000 nM, E100= endoxifen 100 nM. E1000+BE= endoxifen 1,000 nM+ β-estradiol 1 nM, E100+BE= endoxifen 100 nM+ β-estradiol 1 nM, BE= β-estradiol 1 nM.
Observation of MCF-7 cell morphology after four weeks of treatments
Observation of cell morphology through inverted microscope with 40x magnification did not show any morphological changes in MCF-7 cells after four weeks.
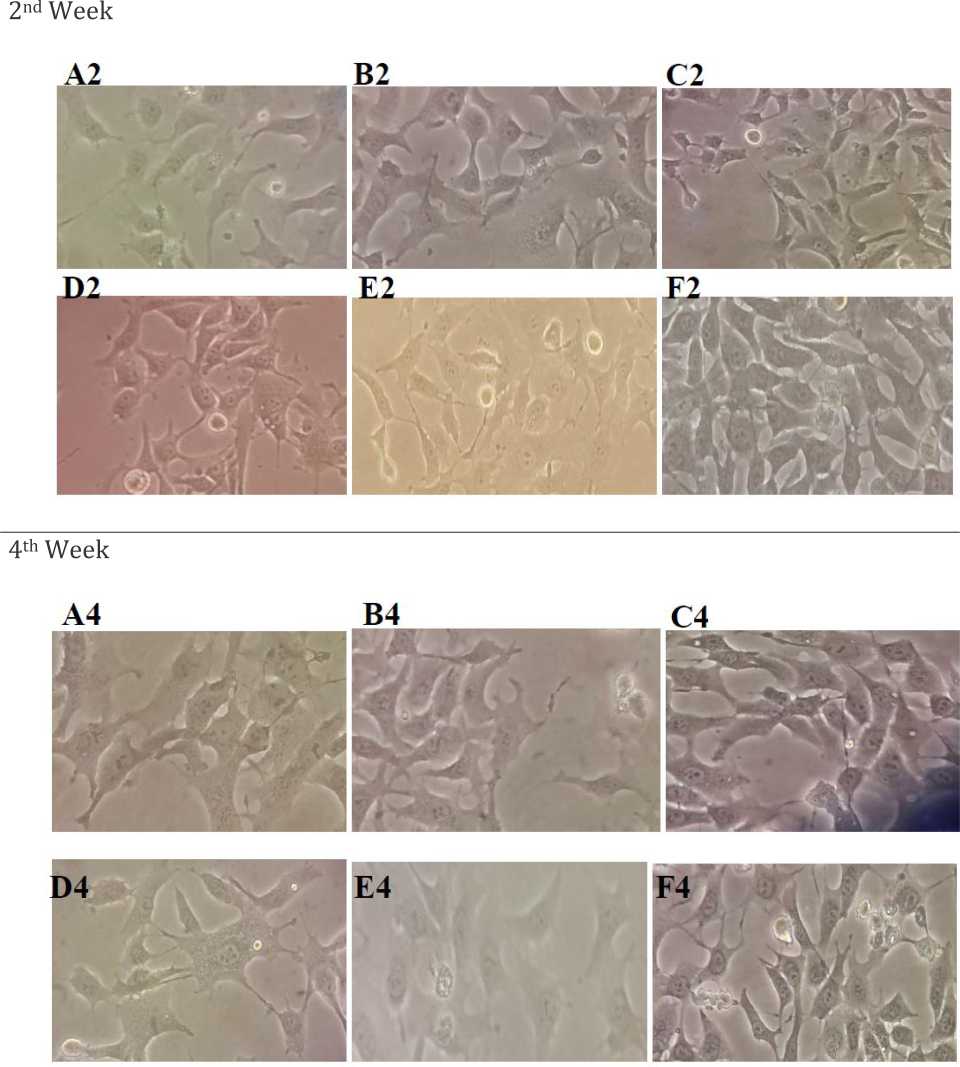
Figure 3. Morphology of MCF-7 cells after seeding at a cell density of 104 viable cells/mL into 6-well plates and grown for four weeks. There was no difference in morphology after four weeks of drug treatment at 40x magnification using inverted microscope. A2, A4= endoxifen 1,000 nM, B2, B4= endoxifen 100 nM, C2, C4= endoxifen 1,000 nM+β-estradiol 1 nM, D2, D4= endoxifen 100 nM+ β-estradiol 1 nM, E2, E4= β-estradiol 1 nM, F2, F4= control (DMSO)
The effect of endoxifen on vimentin mRNA expression
The quantitative data of vimentin was normalized to β-actin as reference gene was shown in Figure 4. The results are presented as mean±SD of six experiments in three trials.
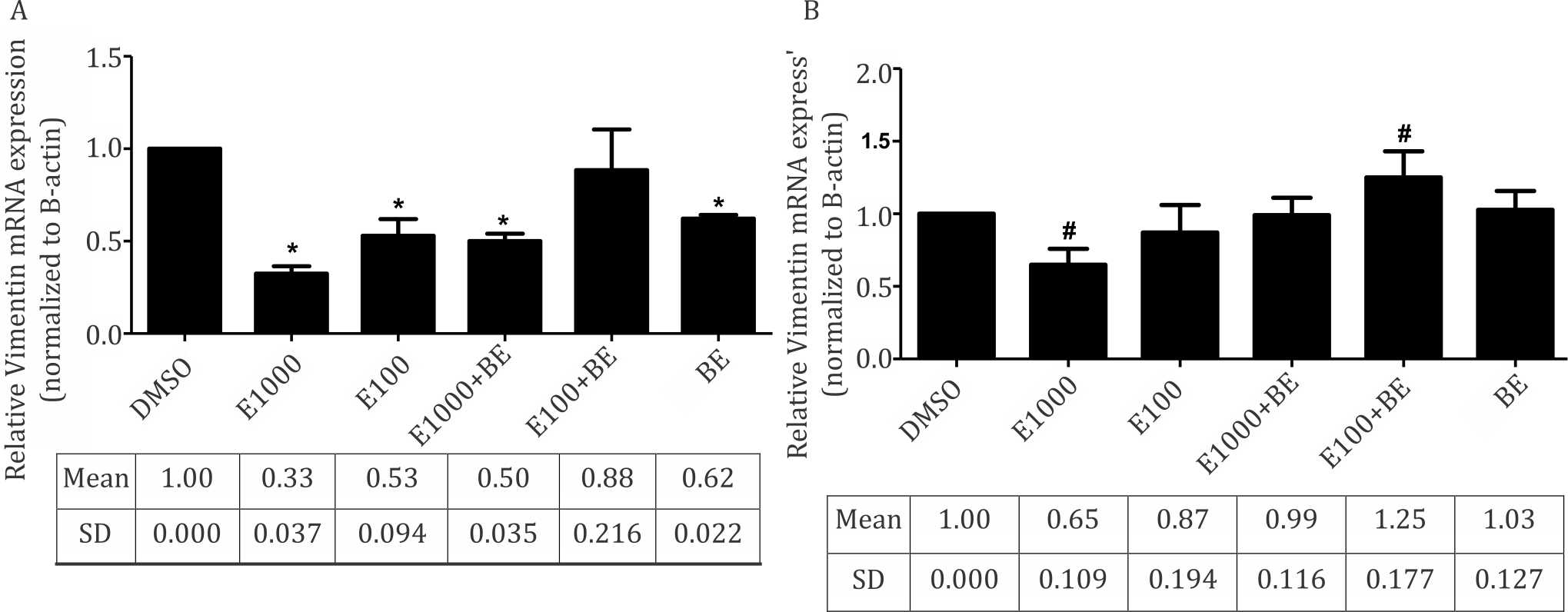
Figure 4. Endoxifen effect with/without β-estradiol on the expression of vimentin mRNA in MCF-7. A=vimentin mRNA expression in second week; B= vimentin mRNA expression in fourth week. *p<0.05 compared to control group (DMSO) after transformation, one-way ANOVA followed by Tukey test
Vimentin mRNA expression analysis showed that both endoxifen 1,000 nM and endoxifen100 nM were able to decrease vimentin mRNA expression to 0.325 and 0.529 fold compared to control group after 2-week of treatment, whereas vimentin mRNA expression level on endoxifen 1,000 nM + β-estradiol and endoxifen 100 nM + β-estradiol were higher than endoxifen treatment without estradiol.
Analysis of vimentin mRNA expression after 4-week of treatment showed that endoxifen 1000 nM and endoxifen100 nM decreased vimentin expression to 0.650 and 0.871 respectively, relative to control, whereas endoxifen 1000 nM + β-estradiol and endoxifen100 nM + β-estradiol showed vimentin mRNA expression levels of 0.990 and 1.251 respectively.
DISCUSSION
This study was aimed to analyze the effect of endoxifen with or without β-estradiol on the mechanisms of EMT in terms of vimentin mRNA expression. The addition of β-estradiol in this study was intended to resemble estradiol levels in breast cancer patient.15
We used very low concentrations of DMSO to dissolve endoxifen and b-estradiol, this is the reason why we chose DMSO as control. DMSO might have toxic and modulatory effects on cells, however, a previous study on MCF-7, RAW-264.7 and HUVEC cells suggested that DMSO up to 0.5% had little or no toxicity.16
One of common methods of establishing resistant cell lines is to use low-dosages intermittent incremental inducement with various and inconsistent dosages.17 However, this method requires a relatively long time. Louie et al18 required six months to make tamoxifen resistant MCF-7 breast cancer cell lines, while Hawse et al3 required 15 months to make endoxifen resistant MCF-7 breast cancer cell lines. In this study, we would like to observe whether short-term exposure of endoxifen, which in this study was four weeks could result in a similar result as the longer one. In this study we did not use increment dosage of endoxifen. A study on drug resistance showed that antibiotic treatment in subtherapeutic levels in the long term would lead to resistance.19
We used high doses of endoxifen (1,000 nM) in our study, based on the result given by Wu et al,1 which showed that low concentrations of endoxifen (20-100 nM) did not affect cell viability in the presence of estradiol in MCF-7 cells.
Analysis of cell viability after 4-weeks of treatment demonstrated that endoxifen 1000 nM was more effective than endoxifen 100 nM in decreasing cell viability. As shown by Wu et al,1 endoxifen 1000 nM could degrade ERα more than endoxifen 100nM. Cells that received estradiol showed an increase in viability compared to the endoxifen only. In our study, increase in cell viability occurred after 2-weeks of endoxifen 100 nM + β-estradiol exposures as compared to DMSO. This result indicated that endoxifen 100 nM was unable to suppress cell viability inducing effect of β-estradiol.
Vimentin mRNA expression analysis showed that endoxifen only (endoxifen100 nM and endoxifen 1000 nM) decreased the expression stronger than endoxifen + β-estradiol. Previous results had shown that addition of β-estradiol could increase vimentin expression. Experiments conducted by Dong et al20 demonstrated that estrogen could induce metastatic potential of thyroid cancer cells by increasing vimentin mRNA expression. Jimenez-Salazar et al21 showed that 1 nM β-estradiol elicit c-Src activation after 15 minutes. The p-Src/ZO-1 complex caused disruption of ZO-1 and ZONAB at the tight junction. In addition, these changes were correlated with the reduced expression of epithelial markers.21 Figure 4 shows that endoxifen 100 nM given concurrently with β-estradiol increased mRNA expressions of vimentin compared to endoxifen 100 nM only. However, the mechanism of this increase has not been clearly elucidated.
Analysis of the mRNA expression of vimentin after 4-weeks of treatment showed that endoxifen with or without β-estradiol relatively increased vimentin expression approximately 2-fold compared with 2-weeks of treatment. Results showed that longer time of exposure had a role in enhancing the expression of vimentin mRNA. Another study reported that administration of endoxifen 1,000 nM for 15 months could induce EMT, which was characterized by the increasing expression of vimentin.
EMT is a process where epithelial cells change into mesenchymal cells that are characterized by increased vimentin expression. Vimentin transfection to epithelial cell, which was conducted by Mendez et al6 showed a change in the shape of cuboidal epithelial cells into mesenchymal cells. These results prove that vimentin has a role in the change in cell shape during EMT. Observations of cell morphology through inverted microscope with a magnification of 40 times showed that there were no morphological changes in MCF-7 cells for four weeks.
Based on the results of this study after four weeks, repeated endoxifen exposure was supposed to induce EMT, which was characterized by a relative increase in vimentin mRNA expression and the addition of β-estradiol could augment mRNA vimentin expression due endoxifen exposure. However, in this study, morphological changes in MCF-7 cells did not occur. This might be due to a relatively short time of drug exposure, as compared to the study by Hawse et al3 (15 months). The vimentin expression in our sudy was still below the control, except for the combination of endoxifen 100 nM + β-estradiol, while the study by Zhao et al22 showed that increase in vimentin expression was about five times in cells treated with estradiol that resulted in morphological changes from cobblestone appearance to spindleshaped cells.
In conclusion, four week endoxifen 1,000 nM exposure was the most effective in suppressing breast cancer cell proliferation. Repeated endoxifen exposure up to four weeks might induce EMT as characterized by the relative increase in vimentin mRNA expressions, while the addition of β-estradiol could augment the expression of vimentin.
Therefore, we suggest that in long-term treatment of endoxifen in breast cancer, regular asessment of resistance marker such as vimentin is needed, so that the treatment can be optimized.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
This research was supported by Postgraduate Research Grant of DRPM UI 2015.
REFERENCES
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69(5):1722–7.
- Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB, et al. Endoxifen’s molecular mechanism of action are concentration dependent and different than that of other anti-estrogens. PLoS One. 2013;8(1):e54613.
- Hawse JR, Subramaniam M, Wu X, Negron V, Muzaffer C, Lingle WL, et al. Abstract PD05-11: development, characterization, and effective in vitro treatment of an endoxifen resistant breast cancer cell line. Cancer Res. 2010;70(24):1.
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–92.
- Satelli A, Li S. Vimentin in cancer and its potential molecular target in cancer therapy. Cell Mol Life Sci. 2012;68(18):3033–46.
- Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24(6):1838–51.
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelialmesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–34.
- Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(7):vii89–92.
- Kalluri R, Weinberg RA. The basic of epithelialmesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
- Wang Y, Zhou BP. Epithelial-mesenchymal transition- -a hallmark of breast cancer metastasis. Cancer Hallm. 2013;1(1):38–49.
- Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers, and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796(2):75–90.
- Kusinska RU, Kordek R, Pluciennik E, Bednarek AK, Piekarski JH, Potemski P. Does vimentin help to delineate the so-called ‘basal type breast cancer’? J Exp Clin Cancer Res. 2009;28(1):118.
- Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol. 2014;50(1):1–6.
- Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA. 2009;106(10):3913–8.
- Folkerd EJ, Lønning PE, Dowsett M. Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol. 2014;32(14):1396–400.
- Jamalzadeh L, Ghafoori H, Sariri R, Rabuti H, Nasirzade J, Hasani H, et al. Cytotoxic effects of some common organic solvents on MCF-7, RAW-264.7 and human umbilical vein endothelial cells. Avicenna J Med Biochem. 2016;4(1):e33453.
- Yan XD, Li M, Yuan Y, Mao N, Pan LY. Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol Rep. 2007;17(5):1163–9.
- Louie MC, McClellan A, Siewit C, Kawabata L. Estrogen receptor regulates E2F1 expression to mediate tamoxifen resistance. Mol Cancer Res. 2010;8(3):343–52.
- Carlet J, Jarlier V, Harbarth S, Voss A, Goosens H, Pittet D, et al. Ready for a world without antibiotics? The pensières antibiotic resistance call to action. Antimicrob Resist Infect Control. 2012;1(1):11.
- Dong W, Zhang H, Li J, Guan H, He L, Wang Z, et al. Estrogen induces metastatic potential of papillary thyroid cancer cells through estrogen receptor α and β. Int J Endocrinol. 2013;2013(941568):1–6.
- Jiménez-Salazar JE, Posadas-Rodríguez P, Lazzarini- Lechuga RC, Luna-López A, Zentella-Dehesa A, Gómez-Quiroz LE, et al. Membrane-initiated estradiol signaling of epithelial-mesenchymal transitionassociated mechanisms through regulation of tight junctions in human breast cancer cells. Horm Cancer. 2014;5(3):161–73.
- Zhao G, Nie Y, Lv M, He L, Wang T, Hou Y. ERβ-mediated estradiol enhances epithelial mesenchymal transition of lung adenocarcinoma through increasing transcription of midkine. Mol Endocrinol. 2012;26(8):1304–15.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id