
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Diastolic function in patients with preeclampsia during pre- and postpartum period using tissue doppler imaging
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i2.1410 Med J Indones. 2016;25:93–7
Received: March 22, 2016
Accepted: May 05, 2016
Author affiliation:
1 Department of Cardiology and Vascular Medicine, Faculty of Medicine, Udayana University, Denpasar, Indonesia
2 Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
I B Rangga Wibhuti
E-mail: rangga.w@gmail.com
Background
Prior studies have compared the E/e’ elevation in preeclampsia patients to normal patients, however there are no data whether this elevation persists after birth. The aim of this study is to analyze diastolic function in preeclampsia patients during pre- and post-partum period using E/e’ parameter measurement.
Methods
This is a prospective cohort study of pregnant women with preeclampsia who were hospitalized and planned for pregnancy termination. Basic clinical characteristics were obtained from all samples. Echocardiography was done prepartum, 48-72 hours after termination, and 40-60 days postpartum. Post hoc analysis using least significant difference method was used to compare the results between measurements.
Results
30 subjects were enrolled in the study. Analysis on E/e’ characteristics showed statistical difference between prepartum E/e’ and 40 days postpartum E/e’ (11.87±3.184 vs 9.43±2.529, p=0.001, CI=1.123-3.751), as well as between 48 hours post-partum and 40 days post-partum period (12.12±2.754 vs 9.43±2.529, p<0.001, CI=1.615-3.771). There were no statistical differences between pre-partum E/e’ and 48 hours post-partum E/e’ (11.87±3.184 vs 12.12±2.754, p=0.633, CI=-1.345-0.832).
Conclusion
This study showed diastolic dysfunction in preeclampsia patients persists up until a few days after birth, but resolves in time (40 days after birth) as measured by tissue doppler imaging.
Keywords
diastolic dysfunction, postpartum, preeclampsia, prepartum
Hypertension is one of pregnancy complications, and the leading cause of global maternal morbidity and mortality. This condition is commonly precipitated by preeclampsia and its comorbidities.1 Preeclampsia occurs when there is overexpression of pro-inflammatory factors, anti-angiogenic factors, and angiogenic factors that cause systemic endothelial cell dysfunction with exaggerated inflammatory response and vasoconstriction.2 Vasoactive hormones play an important role in the pathogenesis of preeclampsia; they are the primary link between placental hypoperfusion, hypertension, systemic complications, as well as proteinuria. In preeclampsia, vasoconstriction and fluid retention occur, thus diastolic dysfunction becomes a significant hemodynamic change in these patients.1
A study compared the diastolic functions in preeclampsia patients using tissue doppler imaging (TDI) parameter (E/e’) in 115 women (first pregnancy) which comprised 52 preeclampsia patients and 63 normotensive patients. The result was an increase of E/e’ value in preeclampsia patients compared to their normal counterpart. This study also found that plasma brain natriuretic peptide (BNP) elevation in preeclampsia patients reflected an increase in cardiac workload, confirmed by elevation in antepartum period and significant decrease in postpartum period.1
Prior studies have compared the E/e’ elevation in preeclampsia patients to their normal cohort, however there are no data whether this elevation persists after birth. The aim of this study is to analyze diastolic function in preeclampsia patients during pre- and post-partum period using E/e’ parameter measurement.
METHODS
This was a prospective cohort study of pregnant women with preeclampsia who were hospitalized and planned for pregnancy termination. Subjects were taken consecutively from Harapan Kita Maternity Hospital and Budi Kemuliaan Maternity Hospital, Jakarta, Indonesia, from April until October 2015. The protocol of this study has been approved by The Committee on Institutional Review Board/Health Research Ethics of national Cardiac Center “Harapan Kita” Hospital (No. LB.02.01/VII/087/KEP.015EV/2016). The exclusion criteria were refusing to participate in the trial after informed consent, prior history of hypertension, diabetes mellitus, smoking, immunological disorder, valvular heart disease or ejection fraction <50%, and/or kidney disease with glomerular filtration rate of <30 mL/min/1.73 m2. Preeclampsia was defined as de novo hypertension followed by significant proteinuria (new onset) of >0.3 g/24 hr. Edema was no longer included in diagnosis criteria, since edema occurs in 60% of normal pregnancy.3
Basic clinical characteristics were obtained from all samples. Echocardiography was done prepartum, 48–72 hours after termination, and 40–60 days postpartum. Echocardiography was performed by an experienced sonographer and validated by an echocardiography consultant. E/e’ parameter was calculated from four chamber views according to American Society of Echocardiography (ASE) guideline.4 Measurement was obtained with volume sample put right on or within 1 cm of mitral valve insertion for e’ wave and adjusted as necessary to stay in the annulus when longitudinal excursion occurs during systolic and diastolic periods. For E wave measurement a 1-mm to 3-mm sample volume is placed between the mitral leaflet tips during diastole, and optimized according to ASE guideline.5
Normality test was done using Shapiro-Wilk test. Post-hoc analysis using least significant difference method was used to compare the results between measurements. Normal distribution data are presented in mean with standard deviation, whereas the abnormal distribution data are presented in median with maximum and minimum value. This study has been approved by the local Institutional Review Board/Health Research Ethics Committee No. LB.02.01/VII/087/KEP.015EV/2016.
RESULTS
Thirty subjects were enrolled in the study. Sample characteristics are presented in Table 1 and 2.
Table 1. Basic characteristics
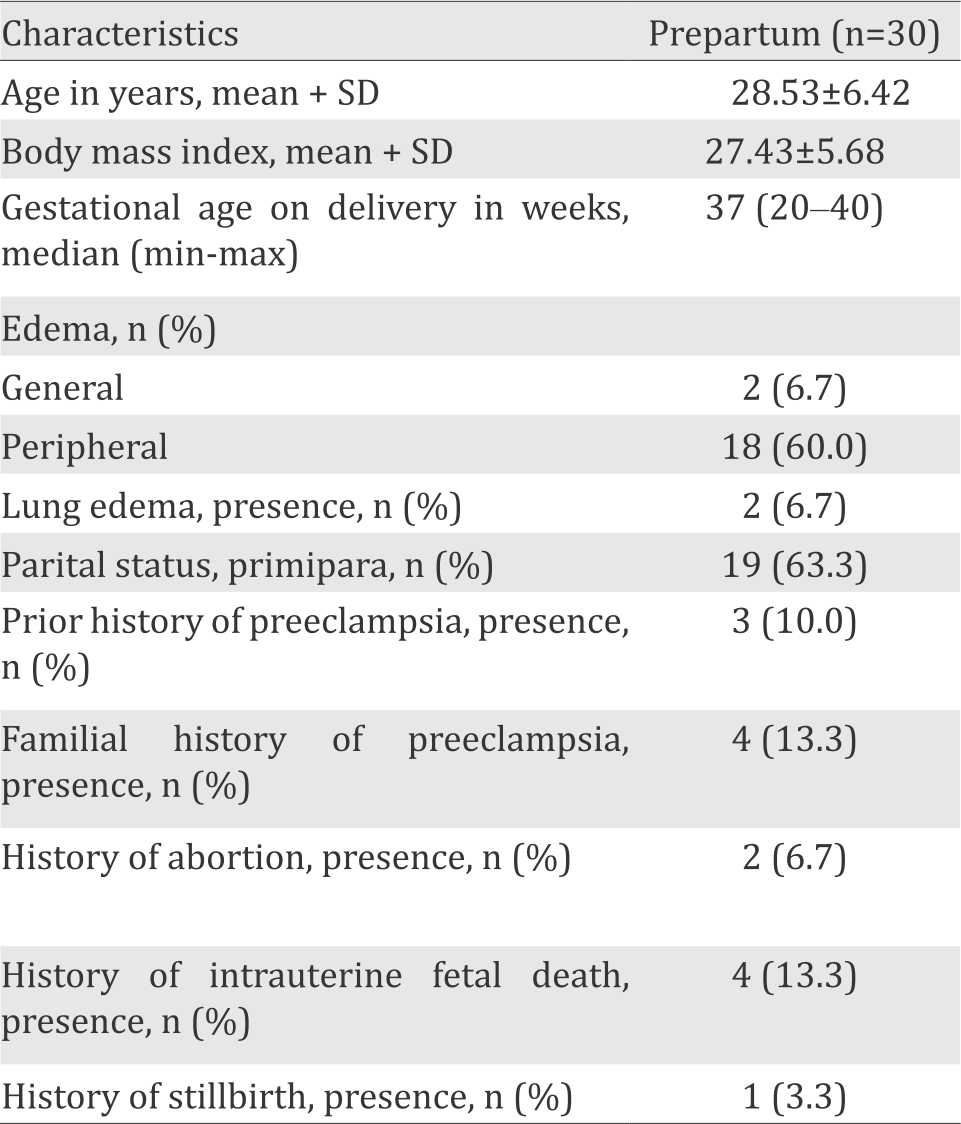
Table 2 shows the difference among characteristics from time to time, prepartum period, 48 hours postpartum period, and 40 days post-partum period. Systolic and diastolic blood pressure as well as heart rate showed a decrease from prepartum period until 40 days postpartum period (p<0.001).
Table 2. Sample characteristics during pre-partum, 48 hours post-partum, and 40 days post-partum periods
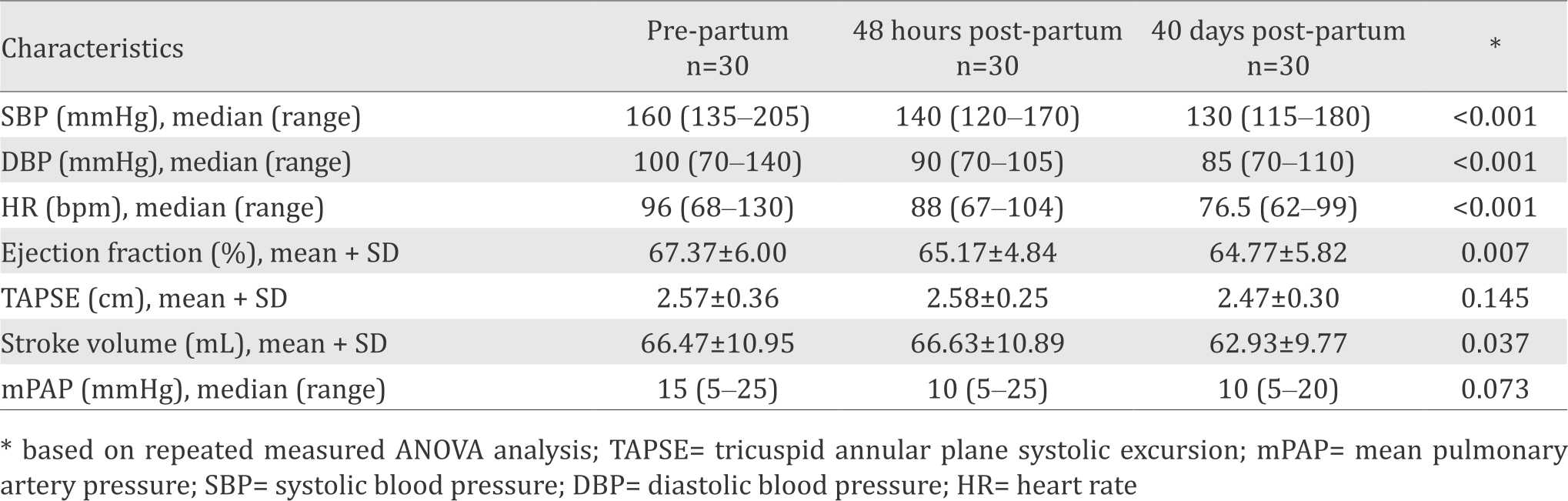
There was significant difference on ejection fraction between pre-partum and 48 hours postpartum period (p=0.005, CI=0.708- 3.692), as well as pre-partum and 40 days postpartum period (p=0.013, CI=0.591-4.609). Tricuspid annular plane systolic excursion (TAPSE) measurements showed no significant difference between each period. Analysis of stroke volume showed a statistical difference between prepartum and 40 days post-partum period (p=0.024, CI=0.502-6.565) as well as between 46 hours post-partum and 40 days post-partum period (p=0.019, CI=0.658-6.742). There is no significant difference in mean pulmonary artery pressure (mPAP) value from time to time (p=0.073).
Normality test on E/e’ data using Shapiro-Wilk’s test showed a normal distribution data (p value of >0.05). Figure 1 provides the data for patients’ diastolic function measured with TDI.
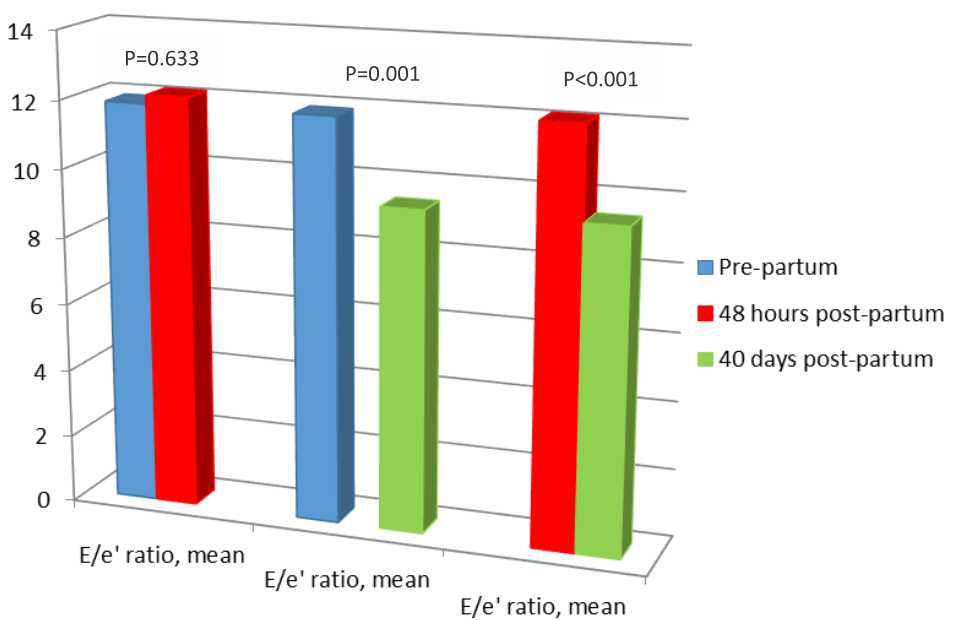
Figure 1. Diastolic function in preeclampsia patients. based on post-hoc analysis test
Analysis on E/e’ characteristics showed statistical difference between pre-partum E/e’ and 40 days post-partum E/e’ (11.87±3.184) vs 9.43±2.529, p=0.001, CI=1.123-3.751), as well as between 48 hours post-partum and 40 days post-partum period (12.12±2.754 vs 9.43±2.529, p<0.001, CI=1.615-3.771). There were no statistical differences between pre-partum E/e’ and 48 hours post-partum E/e’ (11.87±3.184 vs 12.12±2.754, p=0.633, CI=-1.345-0.832).
After adjustment on age, body mass index, edema, lung edema, and heart rate parameters, a significant difference of E/e’ ratio still persists between pre-partum period and 40 days postpartum period (11.87±3.184 vs 9.43±2.529, p=0.001, CI=1.048-3.825), as well as 48 hours post-partum period and 40 days post-partum period (12.12±2.754 vs 9.43±2.529, p<0.001, CI=1.583-3.804). There were no statistical differences between pre-partum E/e’ and 48 hours post-partum E/e’ (11.87±3.184 vs 12.12±2.754, p=0.66, CI=-1.445-0.931) (Figure 2).
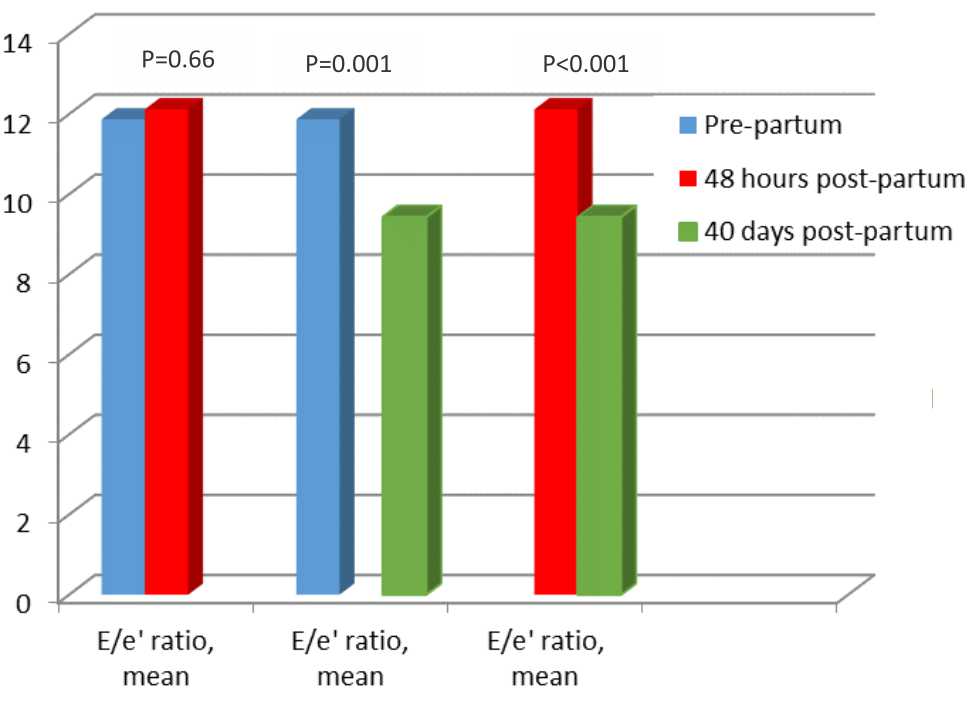
Figure 2. Diastolic function in preeclampsia patients adjusted to age, body mass index, edema, lung edema, and heart rate. based on analysis of covariant to compare differences between measurements
DISCUSSION
E/e’ measurement compared to strain rate examination was proven to have similar accuracy in diagnosing diastolic dysfunction in heart failure patients with normal ejection fraction.6
Normal E/e’ value is influenced by age, e’ value will increase in accordance with age. Normal septal e’ value for 16-20 years of age is 14.9±2.4 cm/s, for 21-40 years is 15.5±2.7, for 41-60 years is 12.2±2.3, and for >60 years is 10.4±2.1.5 Aside from age, body mass index also influences diastolic function, overweight and obese patients independently have negative effects on diastolic function as measured by tissue Doppler imaging.7 Previous research also showed that E/e’ parameter is influenced by preload, which in this research is showed by edema: peripheral, general, and lung edema.8 Heart rate frequency also influences E and e’ value.9 Therefore, analysis of covariant was performed adjusted to age, body mass index, cardiac preload, as well as heart rate parameters.
Prior research showed physiologic remodelling during pregnancy such as an increase in left ventricular septal thickness, left ventricular posterior wall thickness, as well as heart chamber size and mass caused by the increase of blood volume during pregnancy (which will increase cardiac pre-load) while systolic and diastolic function measured by global longitudinal strain rate was still normal.10
However, this research showed the presence of diastolic dysfunction in preeclampsia patients during pregnancy, post-partum period, and follow-up period, as measured by E/e’ parameter. This conclusion is supported by previous studies on pregnant women suffering from preeclampsia, where there is an increase of septal E/e’ ratio and lateral E/e’ ratio during pregnancy up until three to six months after delivery.11
These changes may be caused by several reasons. During a normal pregnancy, there is a physiologic remodeling by fetal syncytial trophoblasts which penetrate and remodel maternal arteries, causing them to dilate into large, flaccid vessels. This remodeling accommodates the increased maternal circulation needed for adequate placental perfusion during pregnancy. However in preeclampsia patients this remodeling is somehow prevented, which causes increased left ventricle (LV) wall thickness and mass more pronounced than in normal pregnancy, but less pronounced LV widening.12 This concentric hypertrophy develops secondary to the increased LV workload that accompanies the elevated blood pressure.13 Subsequently impaired diastolic function will occur as shown by increased E/e’ ratio.
Generally once the placenta is delivered there will be a large shift of fluid, but women with severe and early onset of disease may worsen before getting better.12 Even in normal pregnancy, the increase of systolic and diastolic blood pressure still persisted in 48 hours postpartum, and start to return to the same values as those in 12 weeks of pregnancy, at two to six months after delivery.14 This might explained the nonsignificant changes in diastolic function between pre partum and 48 hours postpartum, but there are significant changes between prepartum and 40 days postpartum, and between 48 hours and 40 days postpartum. Other study also showed that the physiologic changes of preeclampsia are completely reversible after delivery.12
In conclusion, this study showed diastolic dysfunction in preeclampsia patients persists up until a few days after birth, but resolves in time (40 days after birth) as measured by tissue doppler imaging.
Conflicts of Interest
The authors affirm no conflict of interest in this study
Acknowledgment
We would like to thank I Wayan Gede Artawan Eka Putra, MD for his help in statistical analysis of this study.
REFERENCES
- Naidoo DP, Fayers S, Moodley J. Cardiovascular haemodynamics in pre-eclampsia using brain naturetic peptide and tissue Doppler studies. Cardiovasc J Afr. 2013;24(4):130–6.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99.
- Regitz-Zagrosek V, Lundqvist CB, Borghi C, Cifkova R, Ferreira R, Fo25-2-1410 JM, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–97.
- Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33.
- Kasner M, Gaub R, Sinning D, Westermann D, Steendijk P, Hoffmann W, et al. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur J Echocardiogr. 2010;11(9):743–51.
- Kossaify A, Nicolas N. Impact of overweight and obesity on left ventricular diastolic function and value of tissue doppler echocardiography. Clin Med Insights Cardiol. 2013;7:43–50.
- Firstenberg MS, Levine BD, Garcia MJ, Greenberg NL, Cardon L, Morehead AJ, et al. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36(5):1664–9.
- Burns AT, Connelly KA, La Gerche A, Mooney DJ, Chan J, Maclsaac AI, et al. Effect of heart rate on tissue Doppler measures of diastolic function. Echocardiography. 2007;24(7):697–701.
- Ando T, Kaur R, Holmes AA, Brusati A, Fujikura K, Taub CC. Physiological adaptation of the left ventricle during the second and third trimesters of a healthy pregnancy: a speckle tracking echocardiography study. Am J Cardiovasc Dis. 2015;5(2):119–26.
- Rafik HR, Larsson A, Pernow J, Bremme K, Eriksson MJ. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J Hypertens. 2009;27(11):2257–64.
- Mammaro A, Carrara S, Cavaliere A, Ermito S, Dinatale A, Pappalardo EM, et al. Hypertensive disorders of pregnancy. J Prenat Med. 2009;3(1):1-5.
- Ghossein-Doha C, Peeters L, van Heijster S, van Kuijk S, Spaan J, Delhaas T, et al. Hypertension after preeclampsia is preceded by changes in cardiac structure and function. Hypertension. 2013;62(2):382–90.
- San-Frutos L, Engels V, Zapardiel I, Perez-Medina T, Almagro-Martinez J, Fernandez R, et al. Hemodynamic changes during pregnancy and postpartum: a prospective study using thoracic electrical bioimpedance. J Matern Fetal Neonatal Med. 2011;24(11):1333–40.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id