
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Effect of zinc supplementation on triglyceride and malondialdehyde levels: study on diabetic Wistar rats induced with streptozotocin
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v27i2.1417 Med J Indones. 2018;27:82–6
Received: April 5, 2016
Accepted: May 6, 2018
Author affiliation:
1 Department of Public Health Nutrition, STIKES Mega Buana Palopo, Sulawesi Selatan, Indonesia
2 Department of Public Health, Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia
3 Department of Clinical Pathology, Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia
Corresponding author:
Resty Ryadinency
E-mail: resty.gizi@gmail.com
Background
Diabetes mellitus is associated with increased blood glucose and triglyceride levels, which can lead to an oxidative stress. Zinc (Zn) is a micronutrient that has antioxidant properties and involved in lipid and glucose metabolism. This study aimed to evaluate the effect of zinc on the levels of fasting blood glucose (FBG), triglycerides (TG), and malondialdehyde (MDA) in male diabetic Rattus norvegicus Wistar rats.
Methods
Diabetes was induced by intraperitoneal (i.p.) injection of 40 mg/kg BW streptozotocin (STZ) and confirmed by FBG level higher than 200 mg/dL after 2 weeks. The rats were randomly divided into three groups: control group (STZ), treatment I (STZ + zinc 5 mg/kg BW), and treatment II (STZ + zinc 10 mg/kg BW). Zinc was administered by oral gavage for 30 days. At the end of the experiment, levels of FBG, TG, and MDA were measured. Data were analyzed using paired t-test or Wilcoxon test as appropriate.
Results
Supplementation of 5 mg/kg zinc significantly decreased the levels of FBG (pre-intervention: 328.95±70.90 mg/dl, post-intervention: 144.35±34.27 mg/dl, p<0.05), TG (pre-intervention: 252.48±26.30 mg/dl, post-intervention: 147.90±12.18 mg/dl, p<0.05), and MDA (pre-intervention: 12.11±6.46 nm/ml, post-intervention: 4.75±2.34 nm/ml, p<0.05). Moreover, supplementation of 10 mg/kg zinc decreased the levels of TG (pre-intervention: 275.62±56.25nm/ml, post-intervention: 165.58±22.63 nm/ml, p<0.05) and MDA (pre-intervention: 13.08±6.60 nm/ml, post-intervention: 5.08±2.40 nm/ml, p<0.05).
Conclusion
Supplementation of zinc significantly reduced the levels of FBG, TG, and MDA in diabetic rats.
Keywords
fasting blood glucose, diabetes mellitus, MDA, triglycerides, zinc
Diabetes mellitus is associated with disorders of carbohydrates, proteins, and fats metabolism and is caused by a lack of insulin secretion or a decreased sensitivity to insulin.1 World Health Organization (WHO) estimated that 3.4 million people worldwide died of diabetes-associated complications in 2010. At present, 347 million adults worldwide suffer from diabetes. The prevalence of this disease continues to increase, and predicted to be the seventh main cause of death in 2030 worldwide. The prevalence of diabetes in Indonesia is also predicted to increase from 8.4 million in 2000 to 21.3 million in 2030.2 Diabetes mellitus is characterized by hyperglycemia and is associated with an increased free radical activity. Hyperglycemia induces the production of reactive oxygen species or excessive free radicals, which lead to an oxidative stress.3 Free radicals are highly reactive chemical species that can cause an oxidative damage to living things by attacking macromolecules, such as lipids, carbohydrates, proteins, and nucleic acids.4 Under normal physiological conditions, a critical balance is maintained between generation of oxygen free radicals and antioxidant defense systems used by organisms to deactivate and protect themselves against free radical toxicity.5
Diabetes mellitus is associated with lipid and lipoprotein metabolism disorders, which depend on the length of the disease and the degree of insulin resistance. Lipoproteins and lipid disorders in diabetes with hyperlipidemia arise from oxidative stress and lead to lipid peroxidation in plasma, tissues, and membranes and tissue damage.6 One of the disorders associated with lipid metabolism is characterized by serum triglyceride level >200 mg/dl.7 Disorders of fat metabolism may also contribute to an increase in oxidative stress.
Lipid peroxidation is the oxidative damage of polyunsaturated fatty acids and is enhanced in response to high levels of lipids, including triglycerides (TG).8 The end products of lipid peroxidation are aldehydes, a hydrocarbon gas, and chemical residues, such as malondialdehyde (MDA). Oxidative stress and lipid peroxidation may affect the complications of diabetes. Administration of antioxidants may protect tissues against free radicals and lipid peroxidation. Zinc is a micronutrient that has antioxidant properties, but the underlying mechanism has not established yet.9 Zinc is one of the most metabolically active micronutrients and functions in endocrine, reproductive, and immune systems and in glucose metabolism. In particular, zinc is required for the adequate formation and function of antioxidants and metallothioneins. Zinc supplementation can control glucose level, correct lipid metabolism, maintain normal blood pressure, and acts as an anti-inflammatory agent.9 Zinc is predicted to have a significant role in normal insulin and lipid metabolism. Zinc also exerts antidiabetic effects in various experimental models and in a limited number of human studies.10 The serum levels of zinc decreased in diabetic rats induced with streptozotocin (STZ) injection twice, and this effect was recovered by zinc supplementation.11 Numerous studies were conducted to clarify the molecular mechanisms underlying the action of zinc in diabetes to help develop targeted therapy and guide future research. 12 In this study, we investigated the effects of zinc sulfate (5 and 10 mg/kg) on the levels of fasting blood glucose (FBG), TG, and MDA in STZ-induced diabetic rats.
METHODS
Subject
Twenty-four male Wistar rats aged 8–12 weeks and weighing 130–223 g were used in this study. The rats were maintained in polyethylene cages in a laboratory with controlled ambient temperature (22°C–23°C) under a 12 h light–dark cycle and given with standard animal food Comfeed AD II and water available ad libitum. All research and animal care procedures were approved by Research Laboratory and Integrated Testing Universitas Gadjah Mada, Yogyakarta. The rats were allowed to acclimatize to the laboratory for seven days before the experiment. The study protocol was approved by the Medical Research Ethics Committee, Universitas Diponegoro. The method of data collection was approved by the Medical Research Ethics Committee with number 659/ EC/FK-RSDK/2014.
Diabetes induction
The animals were fasted for 6 h but given free access to water prior to STZ induction. The rats were given with single intraperitoneal injection of 40 mg/kg BW freshly prepared STZ. Hyperglycemia was confirmed by elevated glucose levels in the plasma of rats fasted for 12 h and determined on day 14 after STZ injection.
Treatment
Twenty-four male Wistar rats were divided into three groups (n=8 each group). Group I (control) received STZ, group II received STZ followed by 5 mg/kg BW zinc sulfate, and group III received STZ followed by 10 mg/kg BW zinc sulfate. Zinc sulfate (1 ml/kg) was given by gavage daily for 30 days.
Biochemistry analysis
Normal and diabetic rats were anesthetized with intraperitoneal injection of a ketamine mixture. Blood (1 ml) was collected and used for measurement of the levels of FBG, TG, and MDA. The blood samples were placed in sterilized tubes and kept at 4°C. The samples were centrifuged to separate clot and serum. FBG level was measured by enzymatic colorimetric GOD– PAP method. Triglyceride level was determined by GPO–PAP method. Plasma MDA level was measured by enzyme-linked immunosorbent assay (ELISA) test kit.
Statistical analysis
Normality test was conducted using Shapiro–Wilk test. Differences among groups were assessed by paired t-test and Wilcoxon test. Values are expressed as mean ±SD. Significance level was set at p<0.05.
RESULTS
Fasting blood glucose
The fasting blood glucose levels were increased by STZ induction. Zinc supplementation reduced the fasting blood glucose levels among the groups (Figure 1).
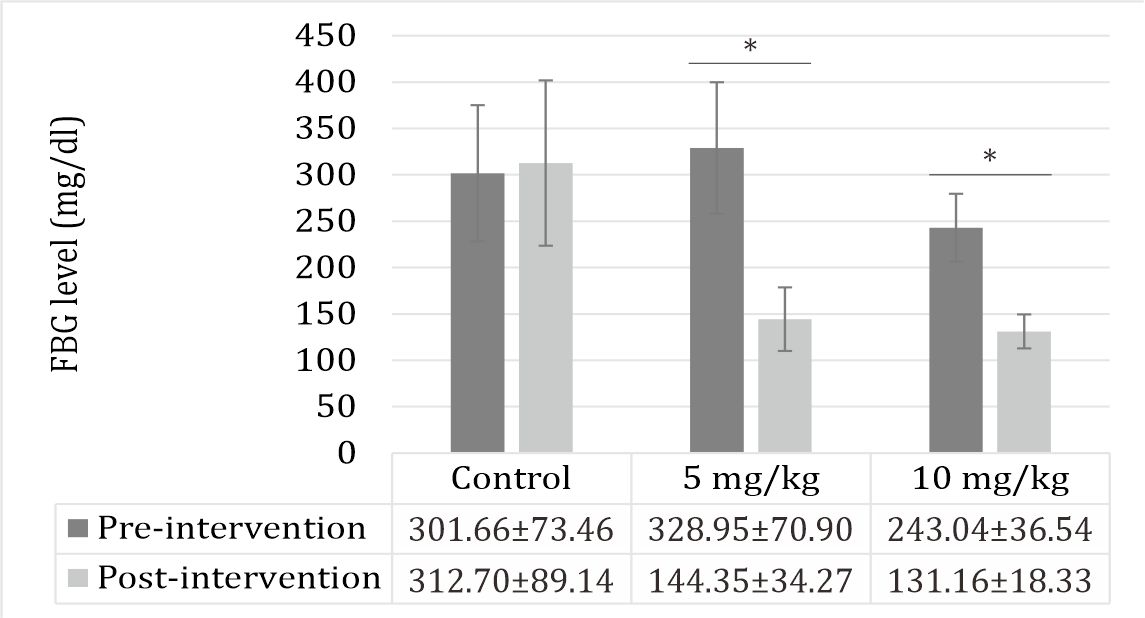
Figure 1. Effect of zinc supplementation on FBG levels. All values are expressed as mean±SD; (p<0.05). Treatments did not affect the fasting blood glucose level.
Triglyceride level
The triglyceride levels were increased by STZ. Zinc sulfate decreased the levels of TG (p<0.05) (Figure 2).
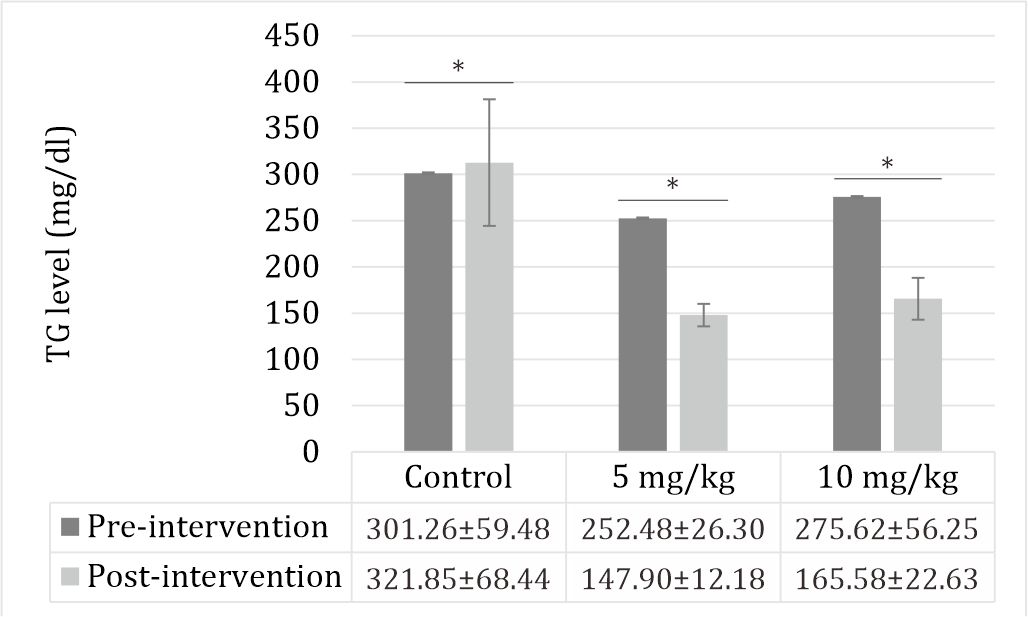
Figure 2. Effect of zinc supplementation on TG levels in STZinduced diabetic rats. All values are expressed as mean ±SD. STZ increased the triglyceride level (p<0.05). Zinc supplementation decreased the triglyceride level (p<0.05).
MDA level
The MDA levels were significantly increased by STZ. Zinc sulfate given at doses of 5 and 10 mg/kg decreased the MDA blood level (Figure 3).
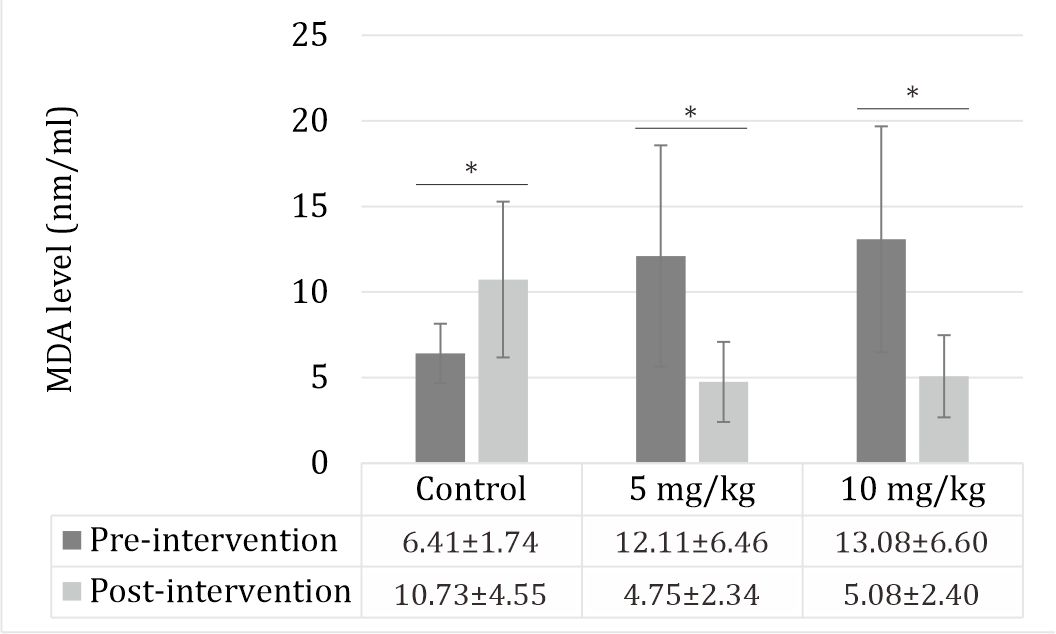
Figure 3. Effect of zinc supplementation on MDA levels in STZ-induced diabetic rats. All values are expressed as mean ±SD. STZ increased the MDA level (p<0.05). Zinc supplementation decreased the MDA level (p<0.05).
DISCUSSION
In this study, zinc supplementation reduced the levels of FBG, TG, and MDA in male Wistar rats with STZ-induced diabetes. STZ is widely used to induce diabetes in a variety of animals because it selectively enhances the degenerative alterations and necrosis of pancreatic β cells, resulting in insulin deficiency and impaired glucose oxidation.13 High blood glucose levels will increase oxidative stress through enzymatic and non-enzymatic processes. In the enzymatic process, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase can disrupt and damage cell function and produces reactive oxygen intermediates, which may oxidize low density lipoprotein (LDL). The non-enzymatic process will alter gene expression (growth factors and cytokines), disturb the antioxidant defense (increased oxidative stress), and lead to failure of β-cell function.14
In STZ-induced diabetic rats, oral administration of zinc threoninate chelate (3, 6, and 9 mg/kg for 7 weeks) reduced the blood glucose levels and increased the serum insulin levels.15 Zinc plays a key role in the synthesis and action of insulin and thus affects the physiological and pathological states of diabetes. Zinc is essential for correct processing, storage, secretion, and action of insulin in pancreatic β cells and is abundant in these cells and their granules.16 Zinc also promotes the phosphorylation of insulin receptors by enhancing glucose transport into cells.17 Zinc exerts insulin-like effects on cells by promoting lipogenesis and glucose transport. Hence, zinc may stimulate tissues to enhance insulin signaling, use glucose, maintain normal lipid metabolism, and maintain normal cellular functions. In this study, the administration of zinc (5 and 10 mg/kg) significantly decreased the levels of FBG. Zinc has been reported to play a key role in regulating insulin production in pancreatic tissues. Therefore, any change in zinc status in the body could affect the production, storage, and secretion of insulin.18
STZ increased the levels of TG, which are the main lipids in fat storage and food. Elevated levels of TG can increase the risk of metabolic syndrome. TG are also associated with insulin resistance and type 2 diabetes mellitus.19 Treatments with zinc (5 and 10 mg/kg) recovered the effects of STZ. Consistent with this finding, a previous study reported that administration of 10 mg/kg zinc in diabetic rats reduced the triglyceride level by 48%.20 Several scholars indicated that zinc-enriched diet has beneficial effects on basal and postprandial glycemia and triglyceride content.21
STZ administration was also associated with increased the MDA level, and this effect was recovered by the treatments with zinc. Living organisms have developed complex antioxidant systems to counteract reactive species and reduce their damage. MDA, an end product of polyunsaturated fatty acids, is a reliable and commonly used biomarker for assessing lipid peroxidation, a well-established mechanism of cellular injury and an indicator of oxidative stress in cells and tissues. In a previous study on male Wistar rats induced by 65 mg/kg STZ, those given with 5 mg/kg zinc for 1 month had decreased MDA levels (p<0.05).22,23 In STZinduced diabetic rats, the oral administration of zinc decreased the plasma MDA levels and improved the antioxidant enzyme activity.15 Zinc is an important component of the body’s antioxidant system and plays a role in retarding oxidative processes related to diabetes mellitus.24
Zinc supplementation controls glucose levels, corrects lipid metabolism, prevents lipid peroxidation, and acts as an anti-inflammatory agent. Zinc supplementation also produces metabolic effects and improves liver function, and nutritional status. Hence, zinc exhibits potential for treatment of type 2 diabetes. Therefore, further studies must be conducted to analyze the zinc level needed to decrease the levels of FBG, TG, and MDA.
In conclusion, this study showed that zinc supplementation decreased the levels of FBG, TG, and MDA in STZ-induced diabetic rats. These effects might be attributed to the antioxidant properties of zinc.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The authors gratefully acknowledge LPPT Gadjah Mada University for their assistance in laboratory. The author himself had born the cost. This study did not receive additional funding from any outside source.
REFERENCES
- Arthur CG, John EH. Text book of medical physiology 11th ed. USA: Elsevier Saunder. 2006. p. 974.
- WHO Media center. Diabetes fact. World Health Organization. 2013. p. 312.
- Moussa SA. Oxidative stress in diabetes mellitus. Rom J Biophys. 2008;18(3):225–36.
- Ramakrishna V, Jailkhani R. Evaluation of oxidative stress in insulin dependent diabetes mellitus (IDDM) patients. Diagn Pathol. 2007;2:22.
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;14(2):231–55.
- Andallu B, Vinay Kumar AV, Varadacharyulu N Ch. Lipid abnormalities in streptozotocin-diabetes: amelioration by Morus indica L. cv Suguna leaves. Int J Diabetes Dev Ctries. 2009;29(3):123–8.
- Shams ME, Al-Gayyar MM, Barakat EA. Type 2 diabetes mellitus-induced hyperglycemia in patients with NAFLD and normal LFTs: relationship to lipid profile, oxidative stress and pro-inflammatory cytokines. Sci Pharm. 2011;79(3):623–34.
- Manohar SM, Vaikasuvu SR, Deepthi K, Sachan A, Narasimha SR. An association of hyperglycemia with plasma malondialdehyde and atherogenic lipid risk factor in newly diagnosed type 2 diabetic patients. J Res Med Sci. 2013;18(2):89–93.
- Sheikhpour R, Jalali B, Yaghmaei P, Afkhami-Ardekani M, Rashidi M. Comparison of two supplementary zinc doses on lipid peroxidation in diabetic patients. Iranian J Diabetes Obes. 2010;2(2):17–22.
- Bicer M, Akil M, Sivrikaya A, Kara E, Baltaci AK, Mogulkoc R. Effects of zinc supplementation on the distribution of various elements in the serum of diabetic rats subjected to an acute swimming exercise. J Physiol Biochem. 2011;67(4):511–7.
- Vardatsikos G, Pandey NR, Srivastava AK. Insulinomimetic and anti-diabetic effects of zinc. J Inorg Biochem. 2013;120:8–17.
- Ranasinghe P, Wathurapatha WS, Ishara MH, Jayawardana R, Galappatthy P, Katulanda P, et al. Effect of zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr Metab (Lond). 2015;12:26.
- Kechrid Z, Derai EH, Layachi N. The beneficial effect of vitamin E supplementation on zinc status, carbohydrate metabolism, transaminases and alkaline phosphatase activities in alloxan diabetic rats fed on zinc deficiency diet. Intl J Diabetes Metab. 2007;15:46–50.
- de Carvalho EN, Ferreira LM, de Carvalho NA, Abla LE, Liebano RE. Viability of a random pattern dorsal skin flap, in diabetic rats. Acta Cir Bras. 2005;20(3):225–8.
- Zhu K, Nie S, Li C, Huang J, Hu X, Li W, et al. Antidiabetic and pancreas-protective effects of zinc threoninate chelate in diabetic rats may be associated with its antioxidant stress ability. Biol Trace Elem Res. 2013;153(1–3):291–8.
- Lin CC, Huang HH, Hu CW, Chen BH, Cheng IW, Chao YY, et al. Trace elements, oxidative stress and glycemic control in young people with type 1 diabetes mellitus. J Trace Elem Med Biol. 2014;28(1):18–22.
- Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol. 2013;27(2):137–42.
- Sheetz MJ, King GL. Molecular understanding of hypergycemia’s adverse effects for diabetic complications. JAMA. 2002;288(20);2579–88.
- Kathleen MB, Peter AM. Metabolisme asigliserol and sfingolipid. In: Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Biokimia harper. 27th ed. Jakarta: EGC. 2009. p. 217.
- Umrani RD, Paknikar KM. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced type 1 and 2 diabetic rats. Nanomedicine (Lond). 2012;9(1):89–104.
- Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc and selenium in healthy subjects. Ann Clin Biochem. 2005;42(Pt 5):364– 75.
- Asri-Rezaei S, Tamaddonfard E, Ghasemsoltani-Momtaz B, Erfanparast A, Gholamalipour S. Effects of crocin and zinc chloride on blood levels of zinc and metabolic and oxidative parameters in streptozotocin-induced diabetic rats. Avicenna J Phytomed. 2014;5(5):403–12.
- Aly HF, Mantawy MM. Comparative effects of zinc, selenium and vitamin E or their combination on carbohydrate metabolizing enzymes and oxidative stress in streptozotocin induced-diabetic rats. Eur Rev Med Pharmacol Sci. 2012;16(1):66–78.
- DiSilvestro RA. Zinc in relation to diabetes and oxidative disease. J Nutr. 2000;130(5):1509S–11S.
Copyright @ 2018 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id