
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Prevalence of Helicobacter pylori among patients with different gastrointestinal disorders in Saudi Arabia
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i4.1442 Med J Indones. 2016;25:214–20
Received: May 09, 2016
Accepted: July 21, 2016
Author affiliation:
Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Shaqra University, Saudi Arabia
Corresponding author:
Mohammed S. Alhussaini
E-mail: malhussaini@su.edu.sa
Background
Helicobacter pylori is an important gastrointestinal pathogen associated with gastritis, peptic ulcers, and an increased risk of gastric carcinoma. The present study was carried out to determine the relationship between this organism with different gastrointestinal ailments.
Methods
150 outpatients referrals to Saudi Arabian Medical City, Riyadh, Kingdom of Saudi Arabia was recruited in January to June 2015. Each patient was subjected to endoscopic examination. Biopsy specimens were taken from the stomach for rapid urease test and culture. Suspected H. pylori colonies were subjected to colony morphology identification, microscopical examination and biochemical reactions. The samples were also subjected to PCR to detect ureA subunit of urease gene.
Results
The endoscopic examination of patients revealed normal, gastric ulcer, duodenal ulcer, gastritis, and gastric cancer with a rate of 20.7%, 20%, 24%, 33.3%, and 2%, respectively. Direct smear exam revealed that 52% of patients were H. pylori positive while culture and rapid urease test showed a prevalence of 71.33%. Fifty four biopsies (36%) were urease positive after 1 hour at room temperature, 39 (62%) after 1 hour incubation at 37°C and 14 (71.33%) after 24 hours incubation. Isolated H. pylori showed that they were catalase, oxidase, and urease positive. PCR results showed 411-bp fragment, which is indicative for the ureA subunit of urease gene.
Conclusion
The prevalence of H. pylori infection was high among tested population. Strong association between H. pylori and duodenal ulcer was noticed. A 411-bp fragment indicative of the ureA subunit of urease gene was detected in all the tested isolates.
Keywords
duodenal ulcer, gastric ulcer, H. pylori, PCR, ureA
Helicobacter pylori (H. pylori) is one of the most common infections in the world. It presents in 70–90% of the population in developing countries and 35–40% in developed ones.1 H. pylori infection is associated with duodenal ulcer,2 gastric ulcer and gastric cancer.3 Approximately, 50% of the normal population across the world harbor H. pylori, though only 10–20% of them become symptomatic.4 There is a high prevalence of H. pylori infection in developing countries especially among children. In India, the prevalence of this infection is 22%, 56%, and 87% in the 0–4 years, 5–9 years and in the 10–19 years age group, respectively.5
A wide range of laboratory investigations are available for diagnosis of H. pylori. The tests are both non-invasive and invasive. Non-invasive tests include, urea breath test, serological immunoglobulin G (IgG), and immunoglobulin M (IgM) detection, saliva and urinary antibody test, and stool antigen test.6 Culture is also a successful method for diagnosis but it is timeconsuming.7 Rapid urease test is a reliable method for presumptive identification of H. pylori8 and enzyme linked immunosorbant assay (ELISA) is the most common tool because of its speed, low costs, simplicity, and reproducibility.9
Polymerase chain reaction (PCR) techniques have also been used for diagnosis of H. pylori. It has widespread use in research as it holds great promise in the detection of genetic differences between H. pylori strains for epidemiological studies. Vinette et al10 showed that PCR is the method with potential for greatest sensitivity and specificity in the detection of H. pylori specific deoxyribonucleic acid DNA. Huang et al11 reported a PCR method based on H. pylori isocitrate dehydrogenase gene sequence that can rapidly and specifically detect the organism. Furthermore, Brooks et al7 developed a PCR assay, which is reportedly 100% sensitive and specific for detection of H. pylori infection in gastric mucosal biopsy specimens. The study aimed to determine the prevalence of H. pylori among 150 outpatients with different gastrointestinal disorders, validate different microbiological diagnostic techniques and detect a subunit of the urease gene (ureA) using PCR to confirm the identification of H. pylori.
METHODS
Patients
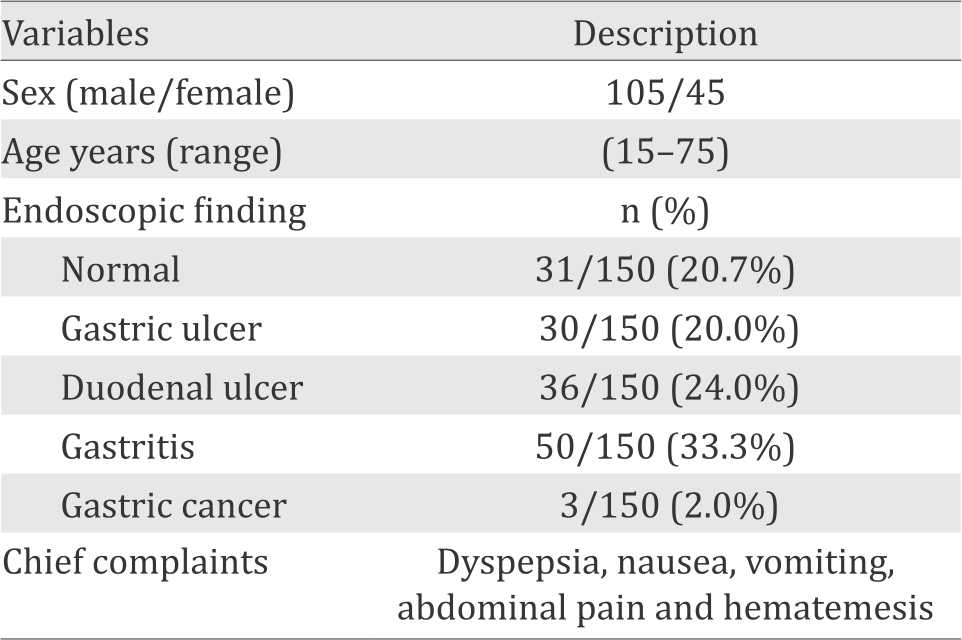
A total of 150 outpatients referrals to Saudi Arabian Medical City, Riyadh, Kingdom of Saudi Arabia with dyspeptic symptoms and clinically indicated for upper gastrointestinal endoscopy were selected. Informed consent mentioning that data from the diagnosis would be used for research purpose was obtained from all the patients. The research protocol was approved by institutional research committee College of Applied Medical Sciences, Shaqra University (53/27844. There were 105 male and 45 female participants with age between 15–75 years. The samples were collected from January to June, 2015. Personal history; name, age, sex, residence, and job were taken from each patient. The main complaints were dyspepsia, nausea, vomiting, abdominal pain and hematemesis. Patients taking antimicrobial drugs and/or bismuth salts within two weeks before endoscopy were excluded from the study.7
Endoscopy
Patients were instructed by the gastroenterologist not to eat or drink for at least eight hours before endoscopy procedure. They received oropharyngeal local anesthetic (Xylocain spray 10%). The endoscope and biopsy forceps were cleansed with Savlon and then sterilized by soaking in Cidexin for 10 minutes followed by washing with sterile water. All endoscopic examinations were carried out by using videoendoscopes PENTAX EPM-3500. Three biopsy specimens were taken from antrum region along the greater curvature of stomach, one was biopsy specimen was used for urease test.12
Culture
The second biopsy specimen was directly smeared and examined for Gram negative curved bacilli. The third biopsy specimen was homogenized and streaked on fresh brain heart infusion agar supplemented with 10% sterile horse serum and on Colombia blood agar with selective supplement (Dent, Oxoid) and modified Columbia agar (MCUA) slant. The plates were incubated under microaerophilic condition using Gas Generating Kits Campylobacter system BR 0546A (Oxoid, Hampshire, England) at 37°C for 3–5 days. H. pylori suspected colonies were identified by Gram stain and biochemical tests. Biopsies were transported in ice from the endoscopic unit to the microbiological laboratory in 1 ml horse serum supplemented brain heart infusion broth13 and processed within two hours.7
Primary diagnosis of H. pylori
The suspected purified colonies were chosen according to the Gram staining and culture's characteristics.
Biochemical tests
Biochemical tests include production of catalase, oxidase, urease, and H2S, nitrate reduction, growing in 3.5% NaCl, growing with 1% glycine, and growing at different temperatures.
Bacterial DNA extraction and purification
Twenty representative samples from pathological cases were subcultured on selective Colombia blood agar and subjected to DNA extraction and purification. The method is described in the protocol of QIAamp DNA Mini Kit® (QIAGENE Inc., Santo Clarita, Calif.), which contains Proteinase K and animal tissue lysis (ATL), cell lysis (AL), wash buffer 1 (AW1), wash buffer 2 (AW2) buffers. Three bacterial colonies were removed from each culture plate with an inoculating loop and suspended in ATL buffer by vigorous stirring. Twenty μl of Proteinase K was added for each sample and incubated at 56°C in incubator shaker until the sample was completely lysed. The tubes were centrifuged briefly to remove drops from the inside of the lid. Two hundred μl of AL buffer was added, mixed by pulse-vortexing for 15 seconds and incubated at 70°C for 10 minutes. The mixture of each sample was applied to the QIAamp spin column. Tubes were centrifuged at 8,000 rpm for one minute. QIAamp spin column was placed in a clean 1.5 ml microcentrifuge tube, carefully opened and 200 μl of AE buffer was added. The mixture was incubated at room temperature for one minute and centrifuged at 8,000 rpm for one minute.
Amplification of DNA by PCR
The oligonucleotide primers used for the
amplification were:
HPU1, (5'-GCC AAT GGT AAA TTA GTT–3') and
HPU2, (5'- CTC CTT AAT TGT TTT TAG –3').
This target DNA sequence was used in developing the PCR assay according to Lu et al14 and He et al15 followed by agarose gel electrophoresis for PCR products. Gel was photographed using Polaroid Pand Camera with an orange lens filter on T 667 Polaroid film.
Statistical analysis
The obtained results were statistically analyzed using MINITAB statistical software, copyright 1992, release 8, MINITAB INC. Chi-square test was applied to calculate the significance difference between various gastrointestinal disorders groups (e.g. gastric ulcer, duodenal ulcer, gastritis, etc).
RESULTS
A total of 150 outpatients with dyspeptic symptoms and clinically indicated for upper gastrointestinal endoscopy were selected. Among these examined patients, the endoscopic findings revealed that 20.7% (31/150) were normal, 20% (30/150) were suffering from gastric ulcer, 24% (36/150) with duodenal ulcer, 33.3% (50/150) with gastritis and only 2% (3/150) with gastric cancer. Table 2 and Figure 1 showed the distribution of these groups according to endoscopic examination. This result revealed that most prevalent finding was patients with gastritis and the lowest those suffering from gastric cancer as shown in Table 1 and Figure 1.
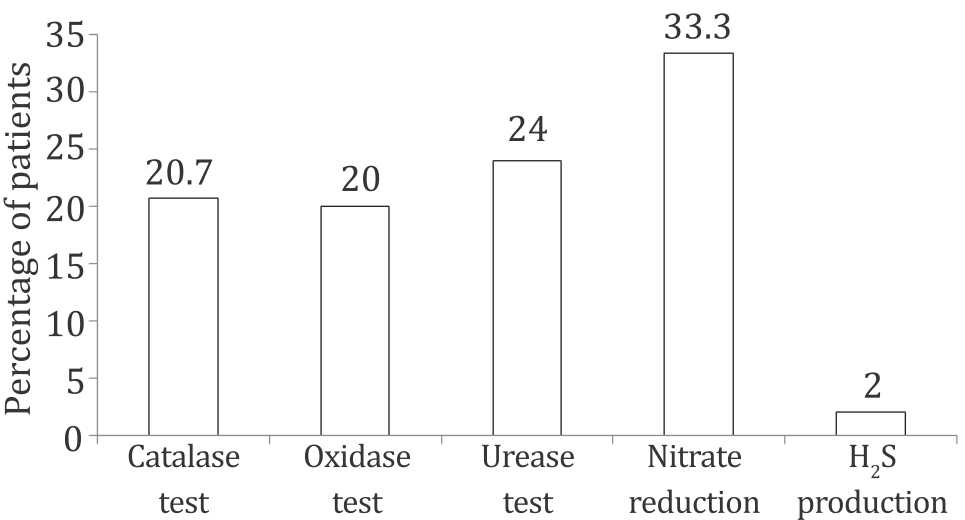
Figure 1. Endoscopic findings in gastric biopsy specimens of studied patients
Table 1. Demographic and clinical characteristics of patients included in the study (n=150)
The culture and biochemical tests showed that 107 (71.33%) out of 150 gastric biopsy specimens were positive for H. pylori. The presence of H. pylori in gastric biopsy specimens, correlated with the change in the color of the slant MCUA tube from orange to pink that occurred at the same time thus giving an additional evidence for the presence of H. pylori in the samples (Figure 2). The colonies of the isolated H. pylori were small to middle in size, rounded, and creamy in color. All H. pylori isolates were Gram-negative spiral to coccobacilli and shared the characteristic catalase, urease, and oxidase production, but differed slightly with respect to other tests (Figure 3). Collectively, some isolates were being positive in nitrate reduction, some were able to grow at 42°C or both. All isolates were unable to grow on 3.5% NaCl, 1% glycin or at 25°C, respectively.
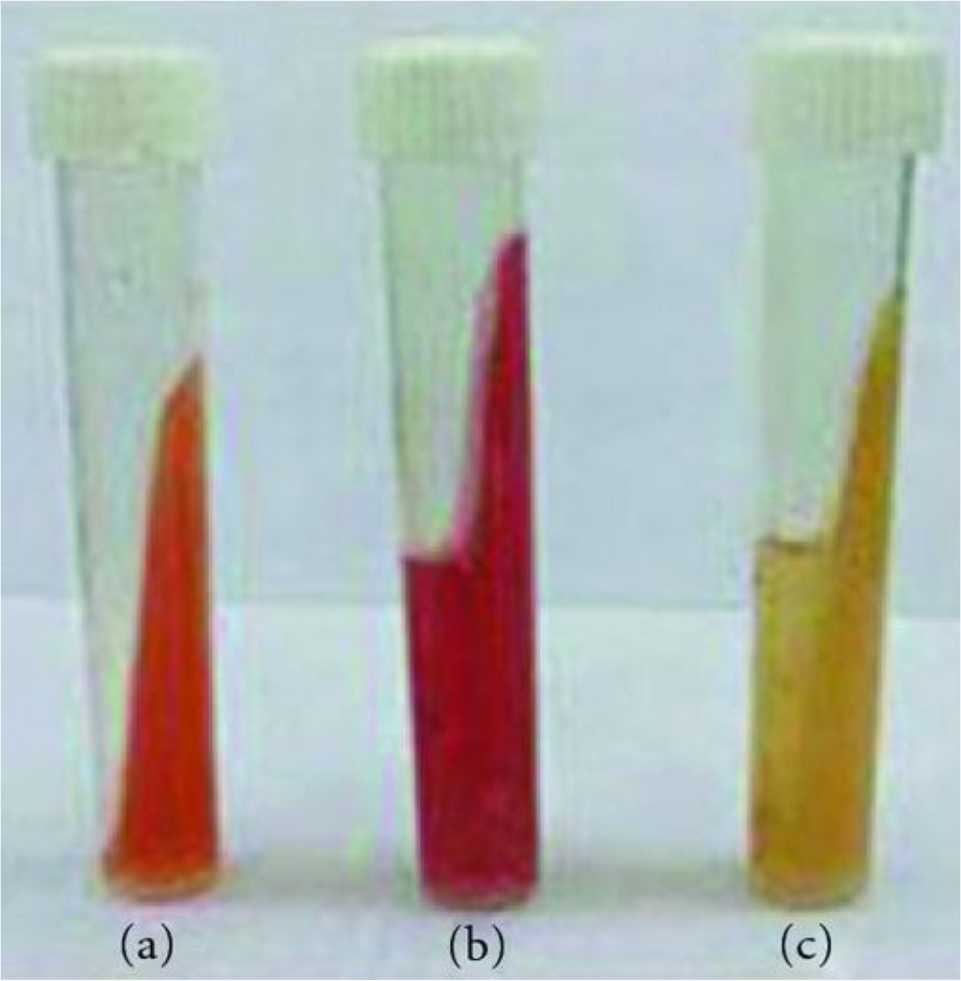
Figure 2. Change in color of slant MCUA tube, a) slant MCUA tube only; b) positive slant MCUA tube culture; c) negative slant MCUA tube culture
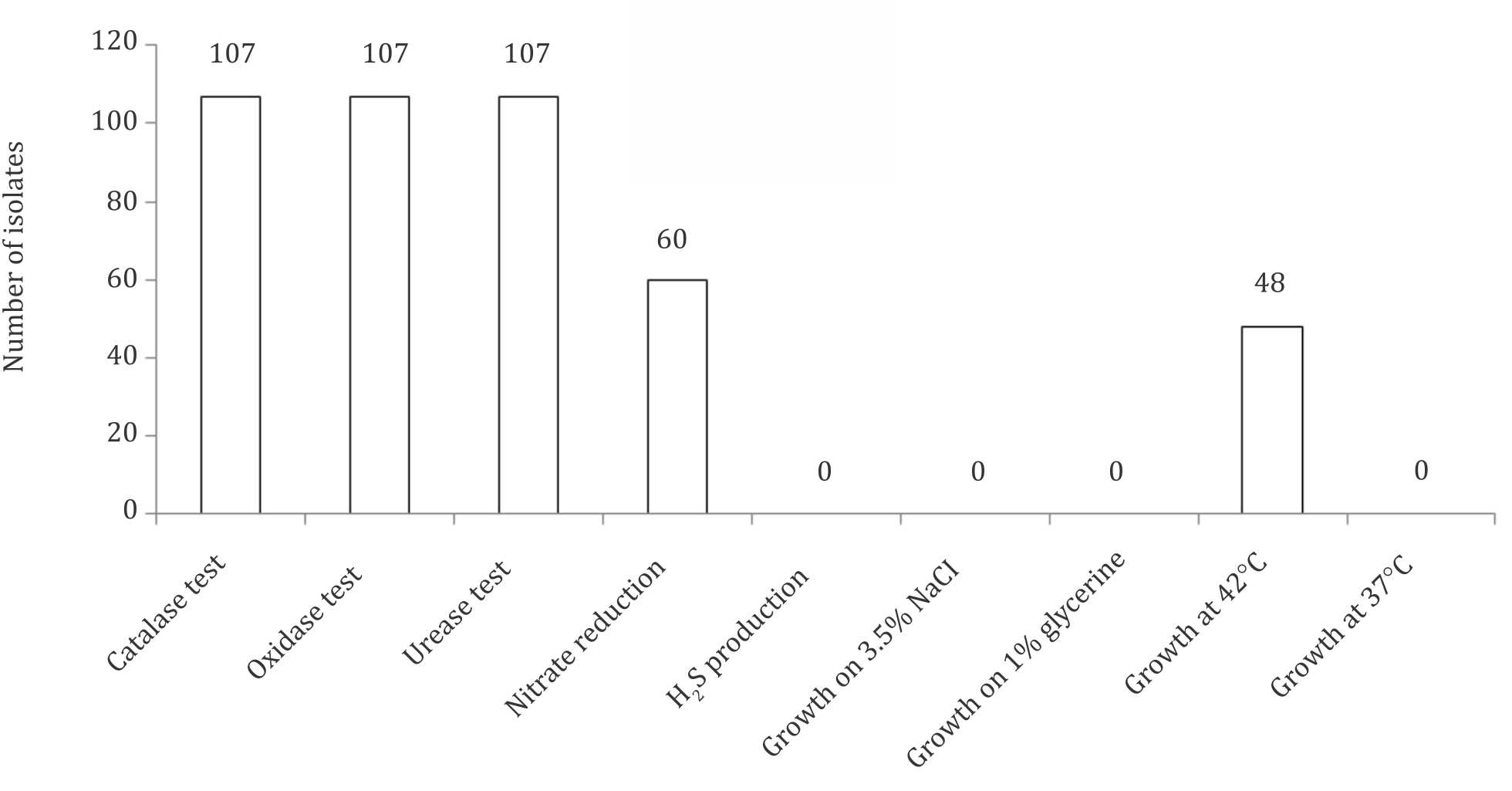
Figure 3. Biochemical tests for identification of H. pylori isolates. *107 isolates were positive for H. pylori out of 150 isolates
The culture and urease test revealed that 107 (71.33%) out of 150 gastric biopsy specimens were positive for H. pylori. The H. pylori was positive in 42% (13/31) of the normal patients, in 63% (19/30), and in 92% (33/36) of patients with gastric and duodenal ulcer, respectively. Seventy eight percent (39/50) of patients with gastritis were H. pylori positive. All patients with gastric cancer were H. pylori positive. The relation between presence or absence of H. pylori and endoscopic findings are shown in Table 2.
Table 2. Frequency of H. pylori detection in gastric biopsy specimens by using rapid urease test and culture
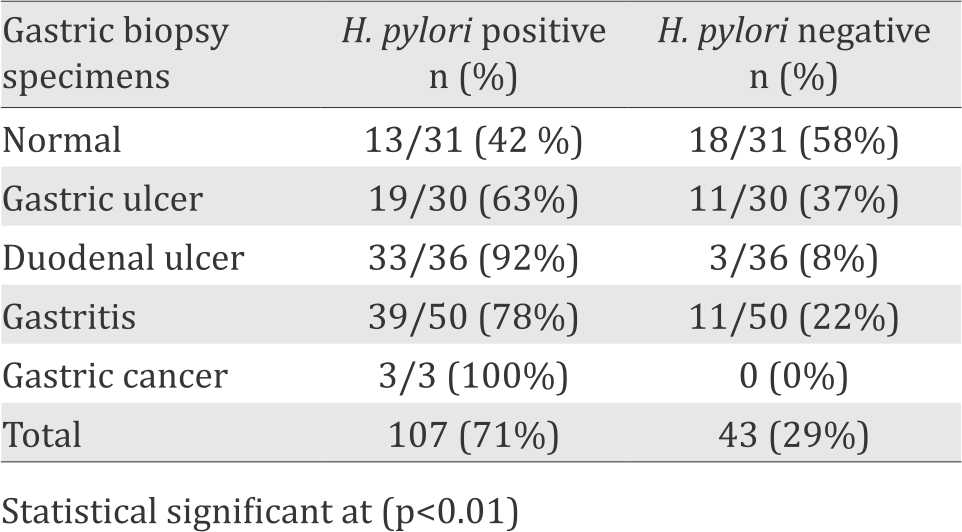
Figure 4 shows similarity between culture and rapid urease test results, while highly significant difference between different gastrointestinal groups with Gram stain results.
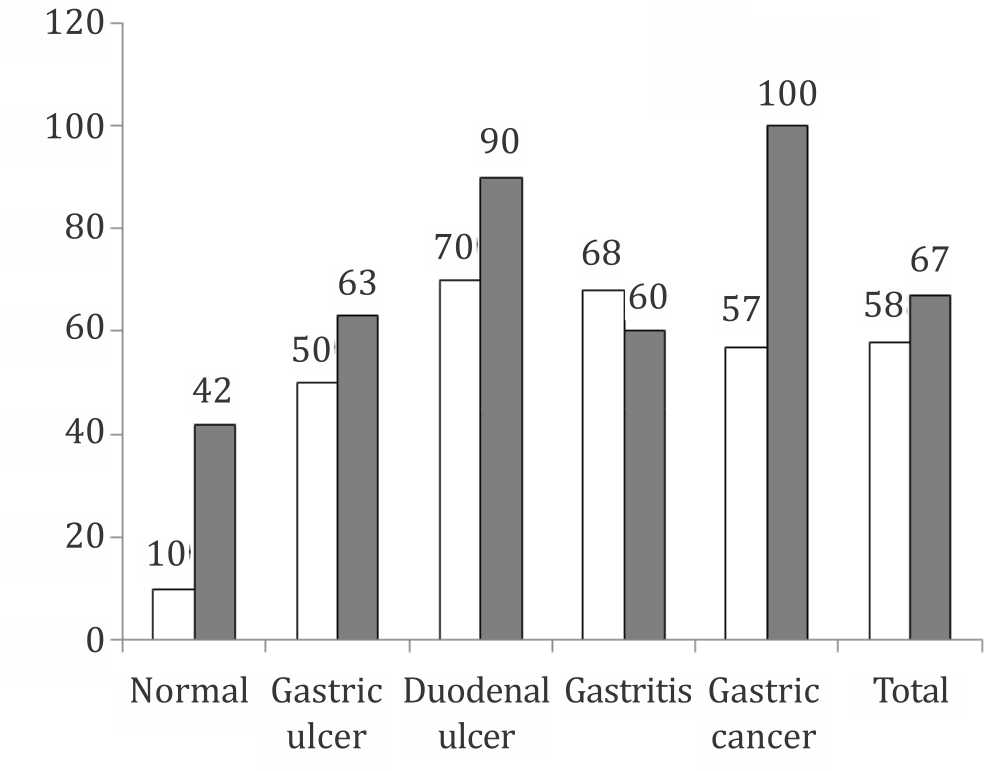
Figure 4. Prevalence of positive H. pylori among studied cases
Samples from 107 isolated H. pylori colonies were subjected to PCR. A 41l-bp fragment indicative of the urea subunit of urease gene was obtained with primers specific for this subunit from these isolated strains. This result confirmed that the isolated colonies were H. pylori. PCR results are shown in Figure 5. Lane 1 shows molecular weight marker (ladder), lanes 2, 4 and 6 are negative controls and lanes 3 and 5 are PCR positive. All of samples were positive for ureA gene by PCR.
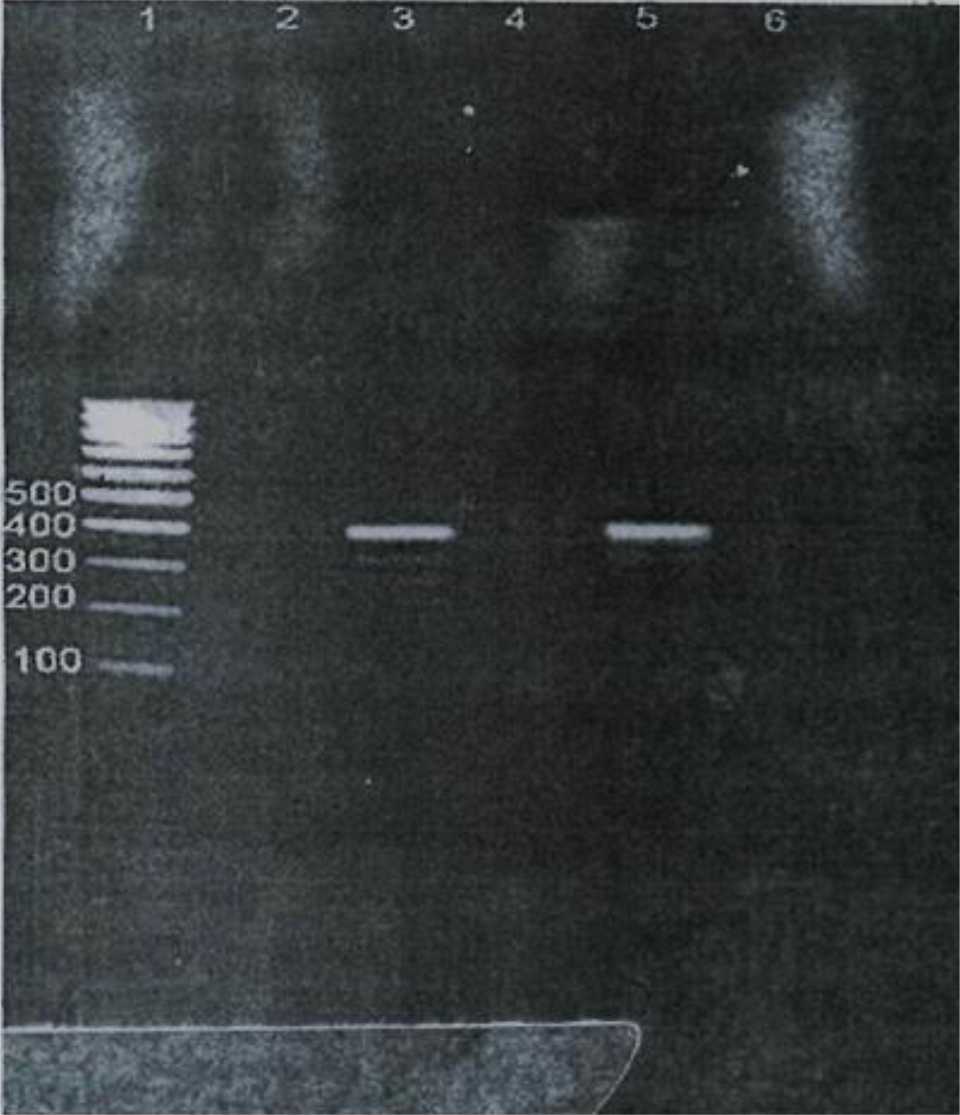
Figure 5. Lane 1 shows molecular weight marker (ladder), lanes 2, 4 and 6 are negative controls and lanes 3 and 5 are PCR positive
DISCUSSION
The isolation rate of H. pylori among dyspeptic patients by culture and rapid urease test was inconsistence with Franzin et al16 who reported that 75.5% of dyspeptic patients were H. pylori positive. On the other hand, our findings were higher than the percentage in the study obtained by Matsukura et al.12 Studies performed at Ismailia (Egypt), 66% and 68%, respectively of dyspeptic patients were H. pylori positive.17,18 A possible explanation for these differences in isolation rates could be attributed to different sample size and methods used for isolation. The present study revealed that incidence of H. pylori among dyspeptic patients with normal endoscopic findings was 42%. This result is similar to the one reported by Suzan18 and Maii17 The incidence of H. pylori among gastric ulcer patients in this study was 63%. This result was relatively around the spectrum of results reported in different parts of the world, which averaged 70%,20 while it was significantly lower than that reported by Franzin16 and Suzan,18 who recorded 78.6% and 75%, respectively. This could be attributed to gastric reflux or due to the effect of analgesic and anti-inflammatory drugs. Furthermore, host characteristics and strain viability may play a role in the pathogenesis of peptic ulcer disease.
Among the duodenal ulcer patients, 92% were H. pylori positive by culture and urease test (Table 1 and Figure 1). This finding is in agreement with Calvet et al20 who reported 95% of duodenal ulcer patients with positive H. pylori. In a similar study, Li et al21 recorded a wide range of positive cases (73-100%). On contrary, Laura et al22 found that duodenal ulcer patients were positive only in 74.3% of studied cases. A strong association between H. pylori and duodenal ulcer was observed in our study.
The present work revealed that incidence of H. pylori among gastritis patients was 78%. This is close to those reported by Suzan18 where isolation rate was 67.5%. Moreover, we detected the organism in all patients with gastric cancer. This result is relatively similar to a previous study which reported 98% of gastric cancer patients were H. pylori positive.11
The results of rapid urease test showed that 71.33% of dyspeptic patients were positive for H. pylori. This finding was in close agreement with previous observations of Suzan18 and Maii17 who reported 66% and 68%, respectively. On contrary, Oyedeji et al23 and Brooks et al7 reported 42.1% and 43.3% positive for H. pylori by urease test. These variations in rapid urease test result may be attributed to certain limitations23 such as the presence of some isogenic urease negative mutants of H. pylori24 or false positive results owing to presence of other urease positive bacteria in the sample.23 Other possible explanation for conflicting results of rapid urease test could be due to reflux of alkaline bile into the stomach that gave false positive result.7 Results of rapid urease test were optimized at different environments. Positive results (36%) were obtained at room temperature after one hour. At 37°C after one hour and 24 hours incubation, 62% and 71.33% positive results, respectively were obtained. The results of this study indicate a good correlation between culture and rapid urease test, as all biopsy specimens diagnosed positive by culture were also positive by rapid urease test. Similarly, the previous findings of Suzan18 and Brooks et al7 showed complete agreement between culture and rapid urease test results.
We used the ure A gene as a PCR target. A PCR product of the anticipated size (411 bp) was obtained from all of samples. Our result is in agreement with He et al15 and Vinette et al10 who reported that ureA gene is conserved and specific to H. pylori. On the other hand, Lu et al14 showed that ureA gene PCR is specific but with low sensitivity (75%), this result was reported when they compared five commonly used primer sets targeting different segments of H. pylori genome. They attributed this low sensitivity to sequence polymorphism in this locus. Brooks et al7 attributed the sensitivity of PCR to the size of the amplified region. They showed that the smaller 294 bp glmM product provides a more accurate result than 411 bp ureA product. These controversial findings in the sensitivity of the target gene may be attributed to different samples as Lu et al14 and Brooks et al7 used gastric biopsy samples while in the current study we used H. pylori colonies directly. Maii17 supposed that different PCR protocols, sampling techniques, and different extraction methods may be a possible explanation for these controversial findings.
To conclude, the prevalence of the infection by H. pylori is high with strong association between H. pylori and duodenal ulcer is noticed. The UreA gene is a strong confirmatory and relevant to H. pylori presence in gastroduodenal diseases.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The author is thankful to all the people who were helpful in collection of data.
REFERENCES
- Duck WM, Jeremy S, Janet MP, Qunsheng S, David S, Friedman C, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10(6):1088–94.
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–5.
- Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302(6788):1302–5.
- Omunakwe HE, Madubuike OC, Nwosu SO, Pughikumo CO, Nwauche CA. Gastric mucosa-associated lymphoid tissue: The need for prompt histologic diagnosis. Ann Trop Med Public Health. 2011;4(2):113–5.
- Das JC, Paul N. Epidemiology and pathophysiology of Helicobacter pylori infection in children. Indian J Pediatr. 2007;74(3):287–90.
- Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut. 2007;56(6):772–81.
- Brooks HJ, Ahmed D, McConnell MA, Barbezat GO. Diagnosis of Helicobacter pylori infection by polymerase chain reaction: is it worth it? Diagn Microbiol Infect Dis. 2004;50(1):1–5.
- Goodwin CS, Armstrong JA. Microbiological aspects of Helicobacter pylori (Campylobacter pylori). Eur J Clin Microbiol Infect Dis. 1990;9(1):1–13.
- Mégraud F. How should Helicobacter pylori infection be diagnosed? Gastroenterology. 1997;113(6 Suppl):S93–8.
- Vinette KM, Gibney KM, Proujansky R, Fawcett PT. Comparison of PCR and clinical laboratory tests for diagnosing H. pylori infection in pediatric patients. BMC Microbiol. 2004;4:5.
- Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–79.
- Matsukura N, Tajiri T, Kato S, Togashi A, Masuda G, Tokunaga A, et al. Diagnostic value of culture, histology and PCR for Helicobacter pylori in the remnant stomach after surgery. Aliment Pharmacol Ther. 2004;20Suppl1:33–8.
- Han SW, Flamm R, Hachem CY, Kim HY, Clarridge JE, Evans DG, et al. Transport and storage of Helicobacter pylori from gastric mucosal biopsies and clinical isolates. Eur J Clin Microbiol Infect Dis. 1995;14(4):349–52.
- Lu JJ, Perng CL, Shyu RY, Chen CH, Lou Q, Chong SK, et al. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J Clin Microbiol. 1999;37(3):772–4.
- He Q, Wang JP, Osato M, Lachman LB. Real-time quantitative PCR for detection of Helicobacter pylori. J Clin Microbiol. 2002;40(10):3720–8.
- Franzin L, Pennazio M, Cabodi D, Paolo Rossini F, Gioannini P. Clarithromycin and amoxicillin susceptibility of Helicobacter pylori strains isolated from adult patients with gastric or duodenal ulcer in Italy. Curr Microbiol. 2000;40(2):96–100.
- Maii AMA. Prevalence of Helicobacter pylori in saliva of patients with chronic gastritis disease as detected by PCR [thesis]. Egypt: Suez Canal University; 2003. p. 85–96.
- Suzan GW. Comparison of serological detection of IgG, culture and rapid urease test for detection of Helicobacter pylori [thesis]. Egypt: Suez Canal University; 2000. p. 45–74.
- Ashton-Key M, Diss TC, Isaacson PG. Detection of Helicobacter pylori in gastric biopsy and resection specimens. J Clin Pathol. 1996;49(2):107–11.
- Calvet X, Sanfeliu I, Musulen E, Mas P, Dalmau B, Gil M, et al. Evaluation of Helicobacter pylori diagnostic methods in patients with liver cirrhosis. Aliment Pharmacol Ther. 2002;16(7):1283–9.
- Li YY, Hu PO, Hazel SL. H. pylori and peptic ulcer disease. Am J Gastroentrol. 1991; 86(4):446–9.
- Laura F, Peitnazio M, Cabodi D, Rossini F, Gioannini P. Clarithromycin and Amoxicillin susceptibility of Helicobacter pylori strains isolated from adult patients with gastric or duodenal ulcer in Italy. Current Microbiol. 2000;40:96–100.
- Oyedeji KS, Smith SI, Arigbabu AO, Coker AO, Ndububa DA, Agbakwuru EA, et al. Use of direct Gram stain of stomach biopsy as a rapid screening method for detection of Helicobacter pylori from peptic ulcer and gastritis patients. J Basic Microbiol. 2002;42(2):121–5.
- Dunn BE, Vakil NB, Schneider BG, Miller MM, Zitzer JB, Peutz T, et al. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect Immun. 1997;65(4):1181–8.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id