Hypercholesterolemia is an established risk factor for cardiovascular diseases that increase the incidence of myocardial infarction and death. Reducing plasma low density lipoprotein (LDL) levels by administering statin therapy has already been proven to reduce morbidity and mortality of cardiovascular disease. The return on expenditure achieved for lipid therapy in Asia (REALITYAsia) study1 demonstrated that only 38% of patients with coronary heart disease managed to reach their LDL targets (at that time, of less than 100 mg/dL). In addition, of the approximately 20 million patients receiving statin therapy, 10–20% of them can not tolerate the drug, mainly due to musculoskeletal side effects.
A landmark study by Cohen et al2 found that the concentration of plasma LDL was determined by pro-protein convertase subtilisin kexin-9 (PCSK- 9). The PCSK-9 is an enzyme produced by the liver, kidney mesenchymal cells, small intestine, and colon epithelial cells. This protein regulates cholesterol homeostasis by binding epidermal growth factor-like repeat A domain of the LDL receptor and leading to a lysosomal degradation. It is demonstrated that a loss-of-function mutation in PCSK-9 gene leads to a lower PCSK-9 plasma level and thus associated with low LDL levels and cardiovascular risk reduction. First discovered in 2003, translational researchs aimed to make PCSK- 9 a target for LDL cholesterol reduction. Several phase-III clinical trials on monoclonal antibodies of PCSK-9 have demonstrated promising results in lowering LDL cholesterol levels.
Structure and regulation of PCSK-9
Pro-protein convertase subtilisin kexin-9 is a member of subtilysin-like serine protease family consisting of 9 members. The first eight members (proprotein convertase -1 (PC1), PC2, furin, PC4, PC5, paired basic amino acid cleaving enzyme-4 (PACE-4), PC7, and subtilysin kexin isozyme-1) break down protein precursors of growth factors, hormones, receptors, and transmembrane transcription factors prior to secretory pathway.3 Instead, PCSK-9 increases endosomal and lysosomal degradation of cell surface receptors that regulate lipid metabolism. PCSK-9 is a glycoprotein composed of 692 amino acids with a molecular weight of 73 kDa. Its structure consists of four domains (prodomain, catalytic domain, cysteine terminal and histidine C-rich terminal) (Figure 1).4
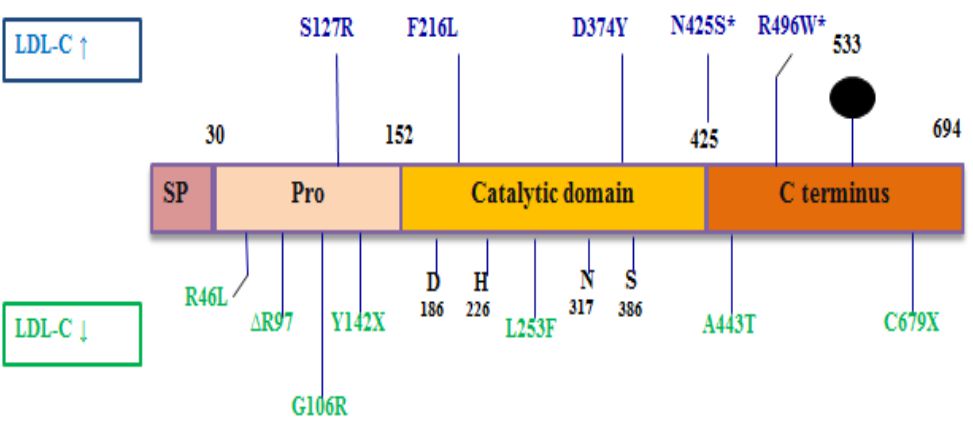
Figure 1. PCSK-9 structure. LDL-C: low density lipoprotein cholesterol. SP: signal peptide. Reprinted with permission from Schulz et al.4
PCSK-9 is synthesized as a zymogen that undergo an autocatalytic breakdown process in the endoplasmic reticulum (FAQ152SIPK site).3 This process is needed for maturation and secretion of protein after emerging from the endoplasmic reticulum. In the extracellular pathway, the PCSK-9 secreted from the transgolgi network, internalize the cell-surface LDL receptors in clathrin-coated endosomes to further degrade them. This process requires cytosolic adaptor binding protein called autosomal recessive hypercholesterolemia (ARH) protein. In the intracellular pathway, PCSK- 9 are exposed directly to the lysosome along with LDL receptors and they become degraded simultaneously.5 The catalytic subunit of PCSK-9 binds to epidermal growth factor (EGF-A) on the human LDL receptor.
Low intracellular cholesterol levels will induce the sterol regulatory element binding protein-2 (SREBP-2) to increase expression of the LDL receptor gene. This increases circulating LDL cholesterol uptake. Meanwhile, SREBP-2 can also induce the expression of PCSK-9 that leads to LDL receptor degradation and thus limits the cholesterol uptake in the liver. This process will prevent excessive cholesterol uptake in order to maintain cholesterol homeostasis.
PCSK-9 and LDL cholesterol
The LDL cholesterol plays a central role in foam cell formation and initiates the process of atherosclerosis. In normal metabolic processes, circulating LDLs undergo hepatic clearance through LDL receptors in hepatocyte cell surface. The mutation of LDL receptor found in familial hypercholesterolemia cases will increase premature cardiovascular events. This increased expression is regulated in transcription level through SREBP-2 and through PCSK-9 and the inducible degrader of LDL receptor (IDOL) after translation.3 PCSK-9 bound to the LDL receptor will thus initiate lysosomal degradation of the LDL receptor meanwhile IDOL will reduce it through lysosomal and poliubiquitination pathway.
Initially, the documentation of the relationships between LDL, LDL receptors, and PCSK-9 level were observed in animal studies. Lagace et al6 found that the injection of pure PCSK-9 resulted in a significant reduction in the LDL receptors on the surface of rat hepatocytes. Mice with high PCSK-9 activity appeared to have a lower number of LDL receptors. Both of these findings were accompanied by low serum LDL levels. Other studies in cultured cells and parabiotic mice also found that PCSK-9 triggered extracelullar LDL receptor degradation through an intermediary adapter protein (ARH) on hepatocytes and lymphocytes.
Previous genetic studies have demonstrated that PCSK-9 had a pivotal role in maintaining cholesterol homeostasis. The gain-of-function mutation of PCSK-9 accelerates atherosclerosis process and leads to manifestation of cardiovascular events. A landmark study by Cohen et al2 compared coronary heart disease (CHD) incidence over 15 year observation in black dan white subjects with non-sense mutation of PCSK-9 (Y142X and C679X). Of the 3,363 black subjects observed, 2.6 percent had nonsense mutations in PCSK-9. This loss-of-function mutation were associated with a 28 percent reduction in mean LDL cholesterol and an 88 percent reduction in the risk of CHD (P=0.008 for the reduction; hazard ratio, 0.11; 95 percent confidence interval, 0.02 to 0.81; P=0.03). Of the 9,524 white subjects observed, 3.2 percent had a sequence variation in PCSK-9 that was associated with a 15 percent reduction in LDL cholesterol and a 47 percent reduction in the risk of CHD (hazard ratio, 0.50; 95 percent confidence interval, 0.32 to 0.79; P=0.003). From this study, it was concluded that moderate lifelong reduction in the plasma level of LDL cholesterol (especially through PCSK-9 inhibition) was associated with a substantial reduction in the incidence of coronary events.
The mechanism of how PCSK-9 may affect plasma LDL levels are currently under investigation. Though previous studies had suggested ARHmediated degradation of LDL receptor by PCSK- 9, this extracellular pathway was not found in fibroblasts.7 This suggests that there are other pathways mediating LDL receptor degradation. An animal study showed that active PCSK-9 can degrade the LDL receptor intracellularly. PCSK-9 binds to the region of EGF-A of the LDL receptors on the cell surface. This bond is stronger in an acidic environment inside endosome. From endosome, LDL receptors will be transported to the lysosome for degradation rather than recycled. These findings prove that PCSK-9 degrades LDL receptors through both extracellular and intracellular pathway.
The promises of PCSK-9 inhibitor
The discovery of PCSK-9 has encouraged experimental and clinical research on lowering LDL cholesterol (LDL-C) levels by targeting it for drug action. Large clinical trials have been conducted to test the efficacy and safety of PCSK- 9 inhibitors. These first-in-class medications are humanized monoclonal antibodies that PCSK-9 acts. That inactivation results in decreased LDLreceptor degradation, increased recirculation of the receptor to the surface of hepatocytes, and consequently lowered the LDL cholesterol levels in the bloodstream.
By 2016, phase III trials had been completed for alirocumab and evolocumab. These phase III clinical trials results were reported in a number of different populations, including patients with statin intolerance, hypercholesterolemic patients on statin therapy, familial hypercholesterolemia subjects (both heterozygous and homozygous), hypercholesterolemic patients who are not taking lipid-lowering medications, and patients on monotherapy compared with ezetimibe (Table 1).
Table 1. Phase III clinical trial results of alirocumab and evolocumab
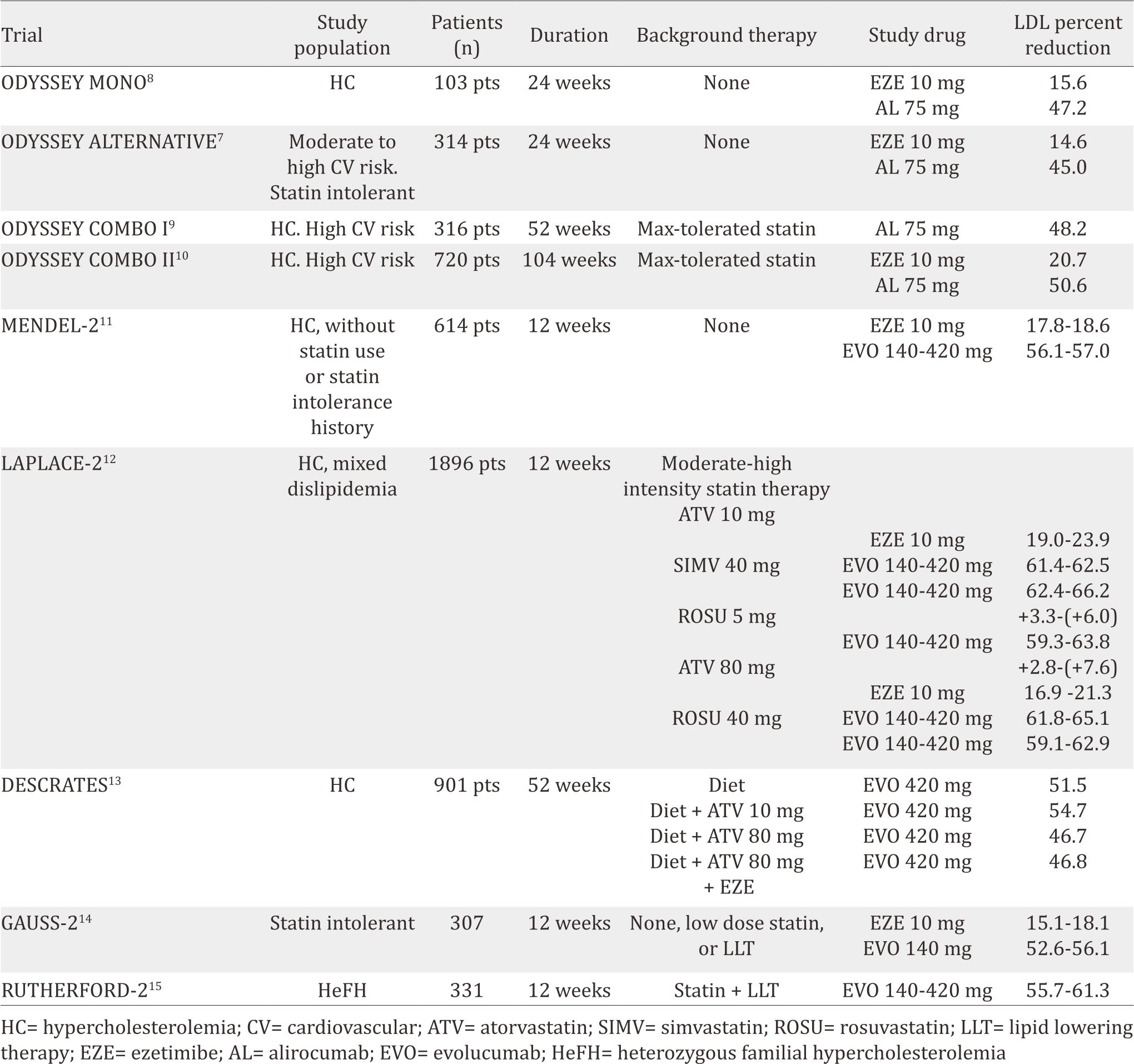
The efficacy and safety of alirocumab (SAR236553/ REGN727) versus ezetimibe in patients with hypercholesterolemia (ODYSSEY MONO)8 trial recruited native hypercholesterolemia patients who had a 1% to 5% 10-year risk of cardiovascular death (scored by European systematic coronary risk estimation). This study included 103 patients, randomized to ezetimibe 10 mg daily or alirocumab 75 mg, injected every two weeks. If LDL-C was ≥70 mg/dL during 8-eight week observation, the alirocumab dose was uptitrated to 150 mg. Results showed that alirocumab reduced LDL-C by 47% compared to 16% by ezetimibe after 24 weeks. Apolipoprotein B (ApoB) and non-high density lipoprotein cholesterol (non-HDL-C) levels were also reduced by 37% and 41%, respectively in alirocumab group. In terms of adverse effect, there were no differences between the alirocumab group and the ezetimibe group. Moreover, evaluation of PCSK-9 inhibitor in patients with statin intolerance was conducted through ODYSSEY ALTERNATIVE trial.7 Three hundred and 14 patients were randomized to alirocumab 75 mg every 2 weeks, ezetimibe 10 mg daily, or atorvastatin 20 mg daily. After 24 weeks of treatment, alirocumab caused 45% LDL reduction compared to 15% in the ezetimibe group. Treatment-emergent adverse events were similar in the two2 groups, with rates of skeletal muscle-related events being lower in the alirocumab than in the atorvastatin group.
In high-cardiovascular risk patients, ODYSSEY COMBO I and II trials tested alirocumab as an additional therapy to statin.9,10 In ODYSSEY COMBO I, 316 patients were randomized to 75 mg alirocumab every two weeks or placebo. Meanwhile, ODYSSEY COMBO II randomized 720 patients to 75 mg alirocumab every two weeks or ezetimibe 10 mg daily. Both trials showed positive results in LDL-C reduction. After 24- week observation, alirocumab reduced LDL-C by 48% while the placebo group only 2% (COMBO I). In the case of COMBO II, the 75 mg alirocumab reduced 51% while the ezetimibe only 21%.
A number of phase III trials were also conducted to evaluate the clinical efficacy and safety of evolocumab. In the monoclonal antibody against PCSK-9 to reduce elevated LDL-C in subjects currently not receiving drug therapy for easing lipid levels-2 (MENDEL-2) study,11 614 patients with hypercholesterolemia were randomized to placebo, ezetimibe, evolocumab 140 mg biweekly, or evolocumab 420 mg monthly for 12-week observation. Evolocumab could reduce LDL-C by 56% to 57% compared to 18% to 19% with ezetimibe without differences in adverse events. The LDL cholesterol assessment with PCSK- 9 monoclonal antibody inhibition combined with statin therapy (LAPLACE-2) study12 with 109 patients also confirmed that evolocumab (evolocumab 120 mg every two weeks or 420 mg once a month) reduced LDL-C by 66% to 75% (biweekly dose) and by 63% to 75% (monthly dose) in the moderate- and high-intensity statin groups compared to placebo. Moreover, biweekly evolocumab could lower non-HDL-C by 58% to 65%, apoB by 51% to 59% and lipoprotein a Lp(a) by 21% to 36% compared to placebo, with comparable reductions demonstrated in the monthly-dose group.
These positive results are consistent with the durable effect of PCSK-9 antibody compared to placebo (DESCARTES) trial findings.13 This trial gives evolocumab 420 mg every four weeks or placebo to 901 randomized hypercholesterolemic patients with LDL-C ≥75 mg/dL and already on atorvastatin 10 to 80 mg daily (with or without ezetimibe). After 52 weeks, evolocumab reduced LDL-C by 57% compared to placebo, ranging from 49% to 62% depending on background lipid-lowering therapy (atorvastatin alone or in combination with ezetimibe). Eighty-two percent of the patients on evolocumab were able to achieve an LDL-C goal of <70 mg/dL. There were no differences in terms of adverse events across groups. In addition, comparison of evolucumab with ezetimibe was also performed in goal achievement after utilizing an anti-PCSK-9 antibody in statin intolerant subjects (GAUSS-2) trial.14 Of 307 randomized hypercholesterolemic and statin intolerant patients, evolocumab 140 mg every twotwo weeks reduced LDL-C by 56% and 55% in the every- two2- weeks and monthly doses, respectively. In addition, evolocumab reduced apoB by 46% and Lp(a) by 24% to 26%.
Moreover, the reduction of LDL cholesterol with PCSK-9 inhibition in heterozygous familial hypercholesterolemia disorder study-2 (RUTHERFORD-2), a multicenter, randomized, double-blind, placebo-controlled trial, recruited 331 heterozygous familial hypercholesterolaemia patients (18–80 years of age) who were on stable lipid-lowering therapy for at least four weeks.15 They randomly allocated in a 2:2:1:1 ratio to receive subcutaneous evolocumab 140 mg every two weeks, evolocumab 420 mg monthly, or subcutaneous placebo every two weeks or monthly for 12 weeks. Compared to placebo, evolocumab led to a significant reduction in mean LDL cholesterol in 12 weeks (every two weeks dose: 59,2% reduction [95% CI 53,4–65,1], monthly dose: 61,3% reduction [53,6–69,0]; both p<0·0001) and at the mean of weeks 10 and 12 (60,2% reduction [95% CI 54,5–65,8] and 65,6% reduction [59,8-71,3]; both p<0,0001). In this study, evolocumab was well tolerated, with adverse events occurrence similar to placebo.
Despite the promising results of many clinical trials available which tested PCSK-9 inhibitors, the price of this new-class drugs are still expensive. For example, alirocumab was launched at a list price of $14,600 per patient per year while evolocumab costs $14,100 per patient per year. Therefore, the cost effectiveness and public health cost efficiency issues need to be considered when prescribing this class of therapy.
In conclusion, advance research in PCSK-9 successfully showed a vital role of this protein in regulating LDL receptors. Suppression of PCSK-9 production was correlated to lower LDL levels. Inhibiting PCSK-9 with PCSK-9 inhibitors has gained attention over the past five years, encompassing positive results of phase-III clinical trials. Nonetheless, cost-effectiveness of this new drug should be evaluated in order to increase public access.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
None.
REFERENCES
- Kim HS, Wu Y, Lin SJ, Deerochanawong C, Zambahari R, Zhao L, et al. Current status of cholesterol goal attainment after statin therapy among patients with hypercholesterolemia in asian countries and region: The return on expenditure achieved for lipid therapy in asia (reality-asia) study. Curr Med Res Opin. 2008;24(7):1951–63.
- Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
- Gu HM, Zhang DW. Hypercholesterolemia, low density lipoprotein receptor and proprotein convertase subtilisin/kexin-type 9. J Biomed Res. 2015;29(5):356–61.
- Schulz R, Schluter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. 2015;110(2):4.
- Urban D, Poss J, Bohm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol. 2013;62(16):1401–8.
- Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, et al. Secreted PCSK9 decreases the number of ldl receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116(11):2995–3005.
- Moriarty PM, Jacobson TA, Bruckert E, Thompson PD, Guyton JR, Baccara-Dinet MT, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statinintolerant patients: Design and rationale of odyssey alternative, a randomized phase 3 trial. J Clin Lipidol. 2014;8(6):554–61.
- Roth EM, McKenney JM. Odyssey mono: Effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol. 2015;11(1):27–37.
- Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The odyssey combo i study. Am Heart J. 2015;169(6):906–15.
- Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The odyssey combo ii randomized controlled trial. Eur Heart J. 2015;36:1186–94.
- Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: The mendel-2 randomized, controlled phase iii clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–40.
- Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on ldl-c lowering in patients with hypercholesterolemia: The laplace-2 randomized clinical trial. JAMA. 2014;311:1870–82.
- Blom DJ, Djedjos CS, Monsalvo ML, Bridges I, Wasserman SM, Scott R, et al. Effects of evolocumab on vitamin e and steroid hormone levels: Results from the 52-week, phase 3, double-blind, randomized, placebo-controlled descartes study. Circ Res. 2015;117:731–41.
- Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The gauss-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–8.
- Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965): 331–40.
