
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Incidence of Candida species colonization in neonatal intensive care unit at Riyadh Hospital, Saudi Arabia
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i3.1444 Med J Indones. 2016;25:171–81
Received: May 09, 2016
Accepted: July 21, 2016
Author affiliation:
Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Shaqra University, Saudi Arabia
Corresponding author:
Mohammed S. Alhussaini
E-mail: malhussaini@su.edu.sa
Background
Candida species are important hospitalacquired pathogens in infants admitted to the neonatal intensive care unit (NICU). This study was performed in the NICU of Saudi Arabian Hospital, Riyadh region, KSA to analyze patterns of neonatal Candida colonization as well as to determine the potential risk factors.
Methods
Weekly surveillance fungal cultures of anal area, oral cavity, umbilicus and ear canal of neonates were performed from birth until their discharge from the hospital. Colonization was analyzed for timing, site, species, birth weight and gestational age. Potential environmental reservoirs and hands of health care workers (HCWs) were also cultured monthly for fungi. Antifungal susceptibility of the identified isolates was also determined.
Results
One hundred subjects have been recruited in this study. The overall colonization rate was 51%. Early colonization was found in 27 (27%) neonates whereas 24 (24%) neonates were lately colonized during their stay in NICU. Colonization was more in preterm neonates than in full and post term. Perianal area and oral cavity were the most frequent colonized sites. C. albicans was the main spp. (58.8%) isolated from the neonates followed by C. tropicalis (17.6%), C. glabrata (15.6%), and C. krusei (2%). Of the 51 isolated Candida spp., 68.6% were sensitive to fluconazole, 80% to itraconazole and 64.7% to ketoconazole, while only 33% were sensitive to amphotericin B.
Conclusion
Candida has emerged as a common cause of infections in infants admitted to NICU, and C. albicans is the most commonly isolated Candidal species. Neonatal infections caused by non- albicans species occur at a later age during their stay in NICU.
Keywords
Candida colonization, neonatal intensive care unit, pediatric patient
Candida is a cause of neonatal infection in premature infants, especially for extremely low and very low birth weight infants.1,2 Candida species have become important nosocomial pathogens in NICUs and are responsible for considerable morbidity and mortality, especially in preterm infants.3,4 Although C. albicans has been the most frequently isolated species in colonized or infected neonates, colonization and infection with non- C. albicans spp., particularly C. tropicalis, and C. parapsilosis, has also increased dramatically.3,5,6 In very low birth weight (VLBW) infants (birth weight less than 1,500 g), C. albicans is the third most common cause of neonatal late onset sepsis (LOS), which occurs after the first 72 hours of life. This was illustrated in a multicenter study from the National Institute of Child Health and Human Development (NICHHD) Neonatal Research Network that evaluated 6,956 VLBW infants (range of birth weight from 401 to 1,500 g) admitted over a two-year period from 1998 to 2000.1 C. albicans was the causative agent in (6%) of first episodes of LOS following coagulase negative staphylococcus (48%), and Staphylococcus aureus (8%) in frequency.2 In addition, C. parapsilosis was isolated as the causative agent in (4%) of the cases.
Transmission of the Candidal strains is either by vertical transmission from the mother or horizontal transmission from health care workers or the hospital environment.7 Colonization with Candida has been identified as the major risk factor and a necessary first step in development of candidemia, providing a reservoir of the potentially invading Candida strains.8,9 Molecular typing studies confirm that most invasive fungal infection arise endogenously and result from a colonizing Candida strain, rather than another completely different isolate.10–12 In view of the risk of colonization, this study aimed to analyze patterns of Candida colonization in the (NICU) of Saudi Arabian Hospital as well as to determine the potential risk factors, the possible source of colonization and the susceptibility pattern of Candida isolates to different antifungal drugs.
METHODS
Cases
This study was conducted on a total of 100 neonates admitted to the NICU of Saudi Arabian hospital (Riyadh region, KSA) from September 2014 to October 2015. Neonates with an expected stay of more than one week in the NICU were enrolled in the study. Fungal surveillance cultures were obtained from the neonates, on admission and at weekly interval thereafter till NICU discharge. Fungal colonization was defined by a positive surveillance culture at any time during their stay in NICU or at baseline.13 Early colonization was considered when Candida species was isolated from the initial cultures, while late colonization was considered when the initial cultures were negative and at least one subsequent culture was positive. Data were collected to assess possible risk factors for Candida colonization such as mode of delivery, gestational age, birth weight (BW), total parenteral nutrition (TPN), use of antibiotics, and other medications.
Samples collection and culture
Pre-moistened (with sterile normal saline) cotton-tipped swabs were used to obtain samples from the oral cavity, perianal area, ear canal, and umbilicus of the neonates’ weekly from birth until discharge. Monthly swabs were also taken from potential environmental reservoirs such as benches, wash basin, and cubicles and from both hands of NICU health care workers (HCWs) rotating through the unit. The entire surface of the hand, the area under the fingernails, and between the fingers, were swabbed with a separate swab for each hand. All the samples were directly inoculated on sabouraud dextrose agar (Himedia laboratories, Mumbai, India) with 50 mg chloramphenicol/liter and 50 mg gentamicin/ liter and incubated at 37°C for 48 hours.
Examination of the mycological growth
Identification of the isolated Candida strains
The conventional yeast identification methods based on morphology, Gram stained smears, sporulation and fermentation characteristics as well as the assimilation of a wide range of carbon and nitrogen sources were used. The isolates were tested to grow on media without different vitamins (thiamine, pantothenate, myoinositole, pyridoxine, niacin, para aminobenzoic acid). The pathogenic potentialities of the yeast isolates were studied by testing protcolytic, lipolytic and haemolytic activity. The species were also determined by the germ tube test and the KJ3006 HiCanclicla Identification Kit (Himedia laboratories, Mumbai, India) according to manufacturer’s instructions.
Confirmatory tests
Tween 80 oxgal-caffic acid (TOC) agar plates were streaked with a 48 hours-old yeast colony, covered with a sterile cover slip, incubated at 37°C for three hours and observed for germ tube production. TOC agar plates were incubated at 28°C for 2–3 days in the dark to promote the production of chlamydospores, hyphae and pseudohyphae. Ascospore formation and urea hydrolysis were also tested for the isolated strains to confirm the identification.
Antifungal susceptibility testing
Susceptibility testing of isolates was performed for amphotericin B, ketoconazole, itraconazole, fluconazole (Hi media laboratories, Mumbai, India) using the disk diffusion method according to the guidelines of Clinical Laboratory Standards Institute (CLSI).14
Statistical analysis
Quantitative variables were presented as medians or mean ± standard deviation (mean ± SD), whereas qualitative variables were described as number and percentages. Data were analyzed using Chi-square tests and Mann-whitney test, as appropriate. Univariate analysis for detection of risk factors was performed using Chi-square tests. A p value of <0.05 was considered statistically significant. All statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) software version.
RESULTS
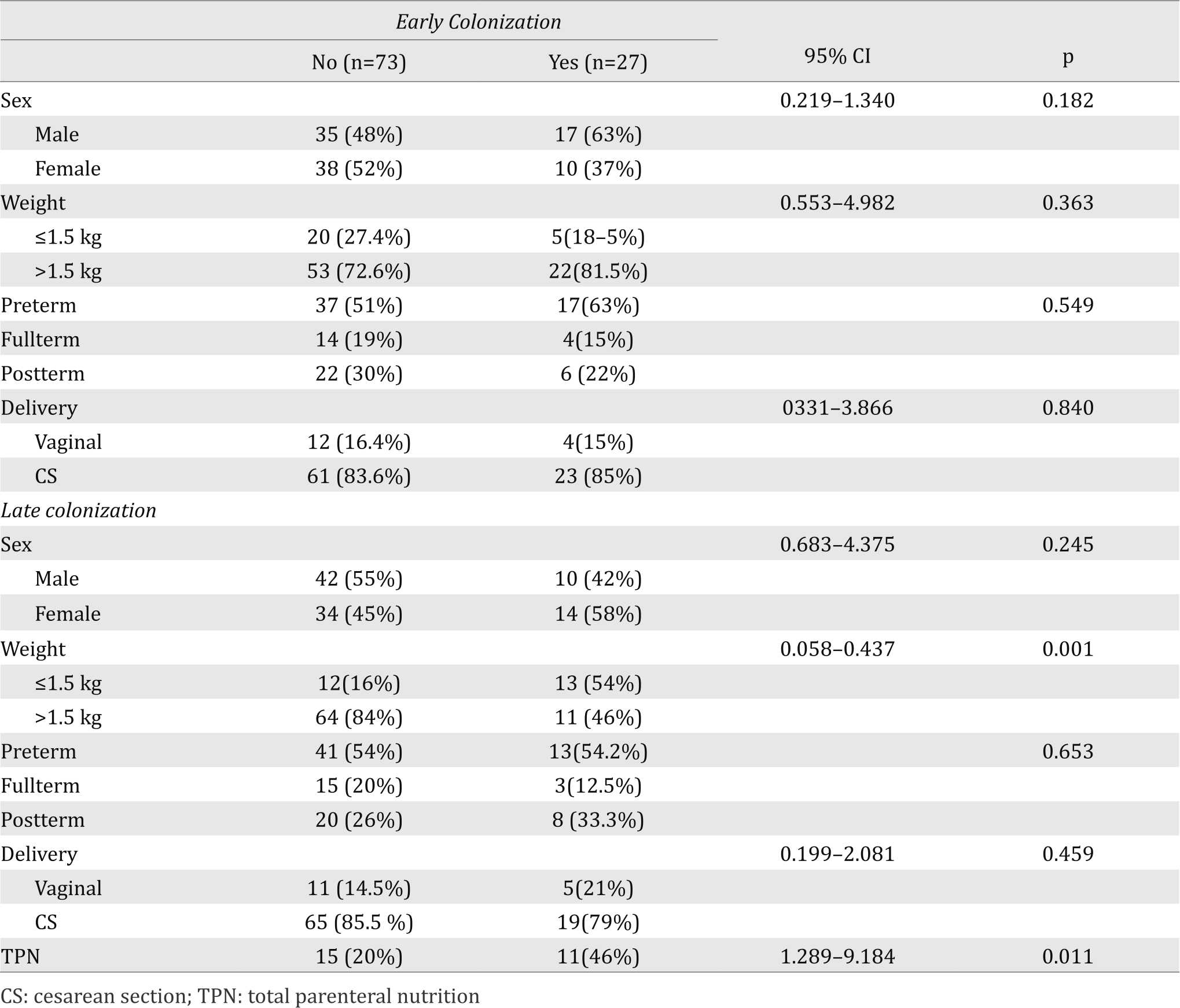
During the study period, Candida colonization was detected in 51 (51%) out of 100 neonates. Early colonization was found in 27 (27%) neonates whereas 24 (24%) neonates were lately colonized during their stay in NICU. Three Candida species were isolated from the hands of 3 nurses on different occasions, while no Candida was isolated from the environmental samples. The characteristics of the neonates and those with both early and late colonization are presented in Table 1.
Table 1. Characteristics of study neonates with early and late colonization
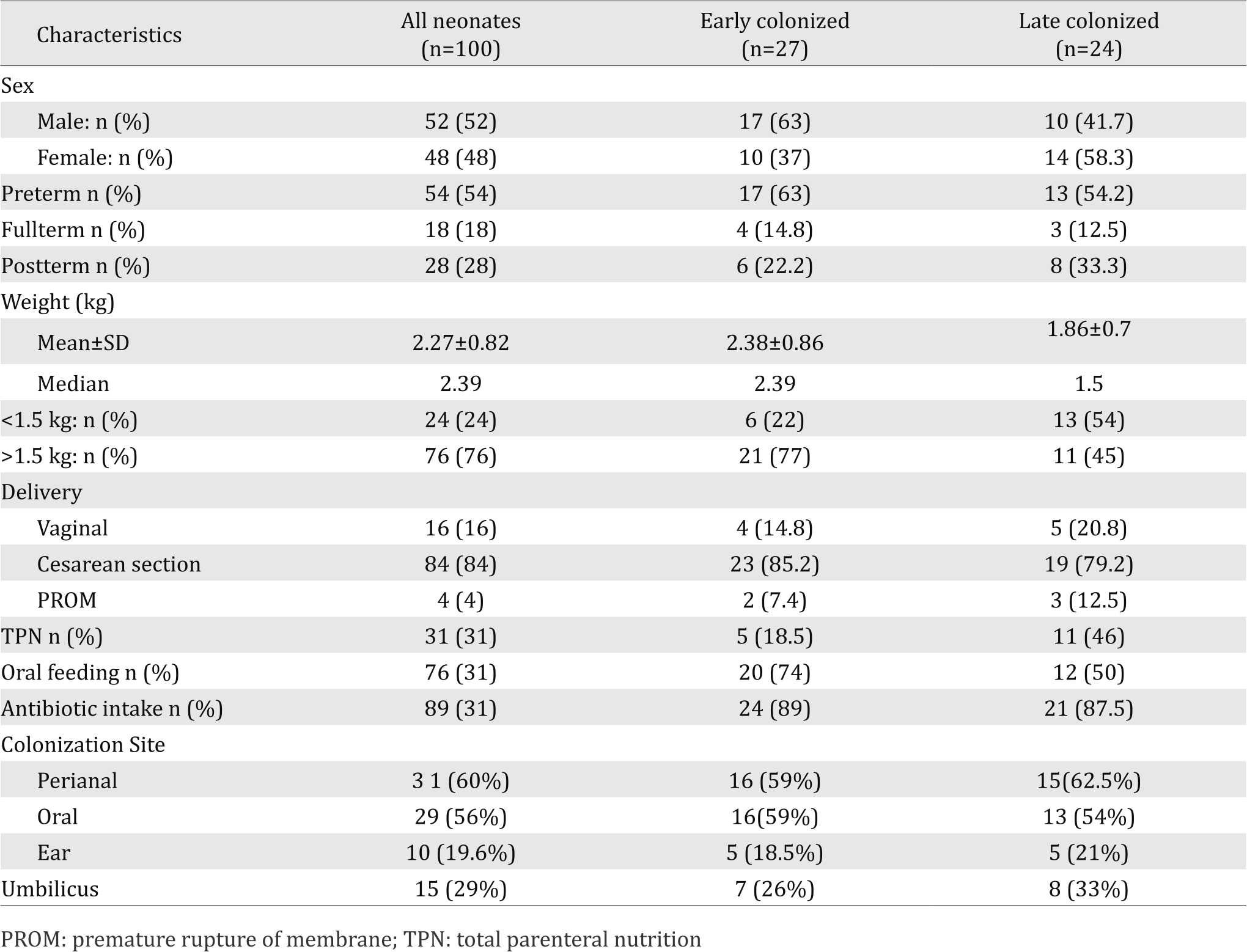
Twenty eight (54%) neonates were colonized with Candida at one site (15 initially and 13 during the stay), while 23 (45%) were colonized at two or more sites (12 initially and 11 during the stay). Perianal area and oral cavity were the most frequent sites of early and late colonization.
Fermentation reactions
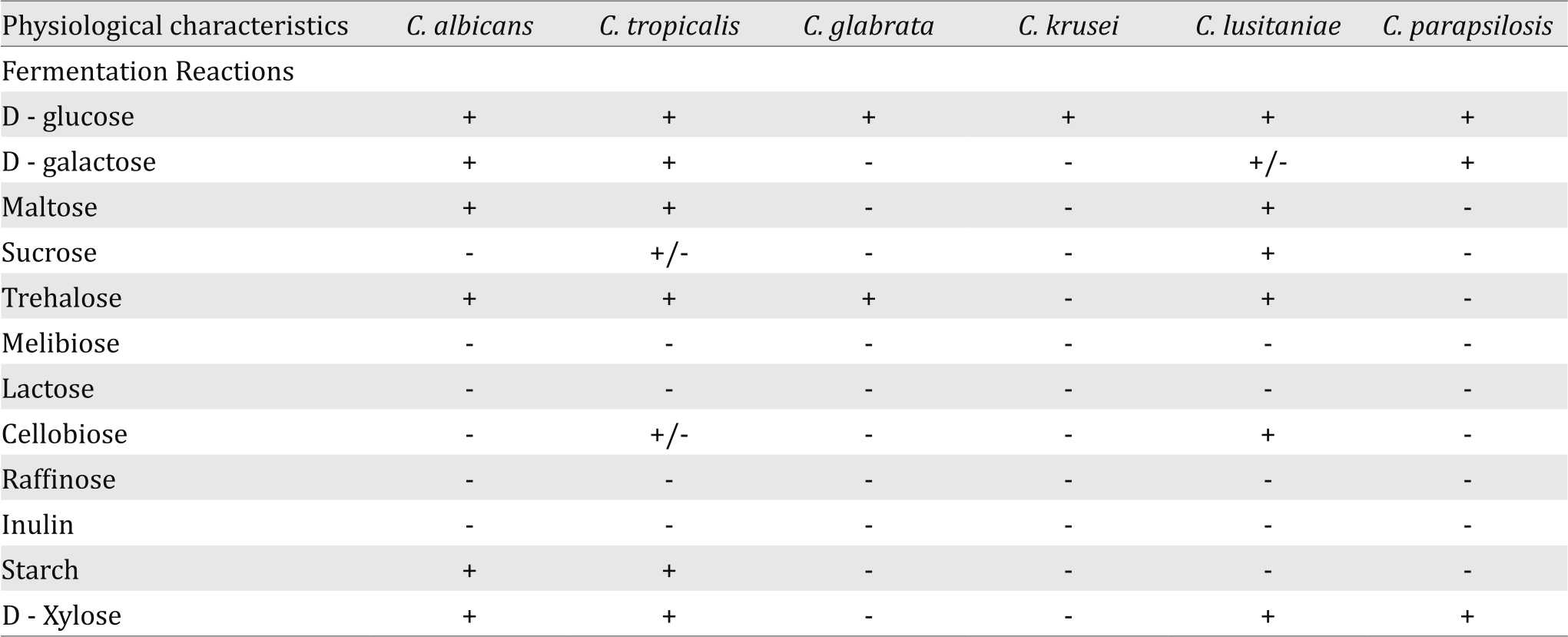
In this study the Candida strains isolated from the study neonates in NICU were also identified by using fermentation tests. The results were negative in all isolates of C. krusei, except Dglucose while the different species of Candida were able to ferment a narrow range of sugars as described in Table 2.
Table 2. Fermentation reactions of Candida strains isolated from the study neonates in NICU
Assimilation tests
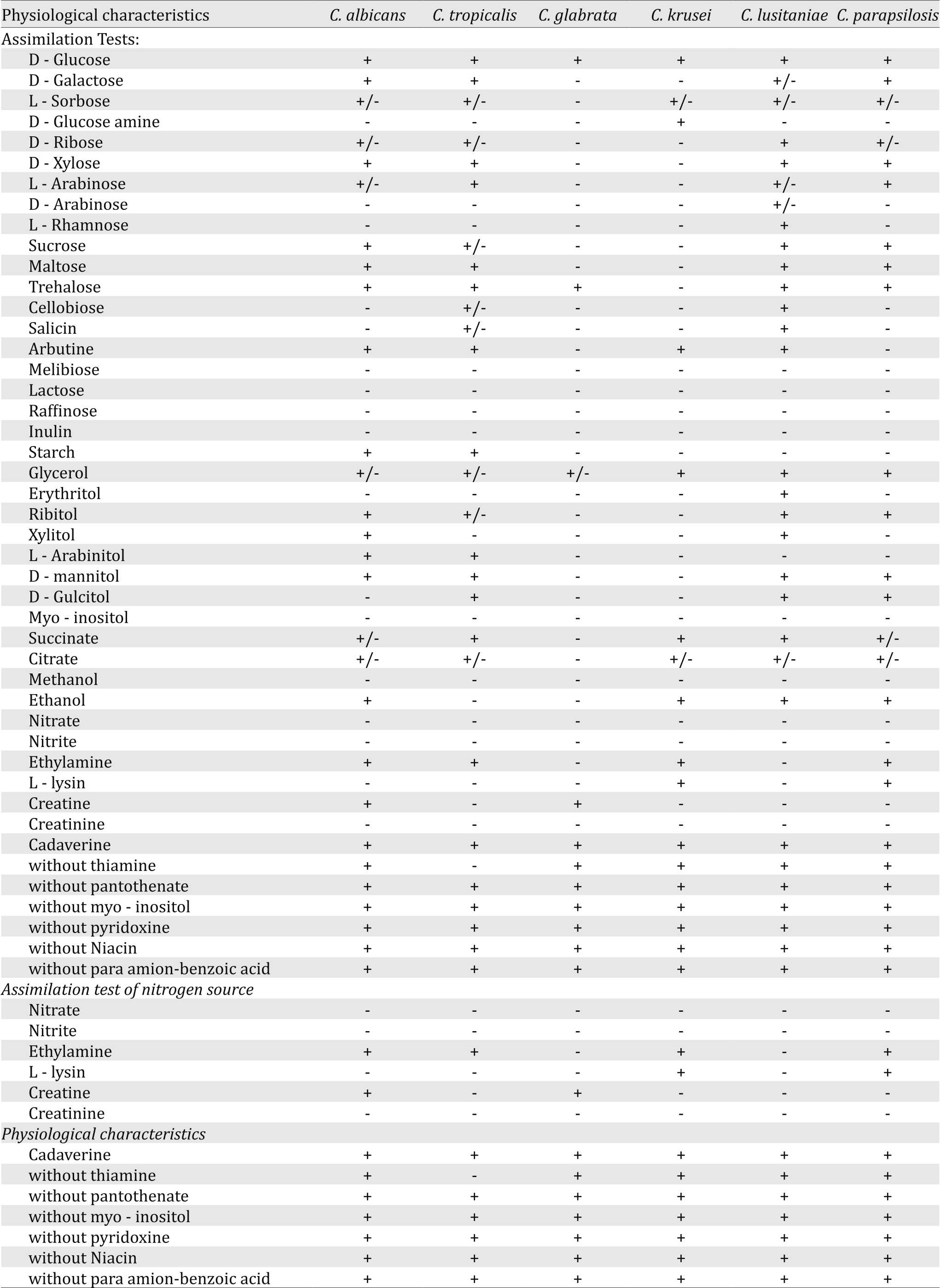
In the present study, six species of Candida were identified; Candida albicans, C. tropicalis, C. glabrata, C. krusei, C. lusitaniae and C. parapsiolosis. The results of vitamin assimilation tests revealed that none of the isolates were capable of growing on melibiose, lactose, erthritol, methanol and inulin as carbon source (Table 3) as well as creatinine, nitrate and nitrite as nitrogen source (Table 3). The results of vitamin assimilation showed that C. tropicalis was unable to grow on medium without thiamine.
Table 3. Assimilation tests of substances as carbon source by Candida strains isolated from the study neonates in NICU
Confirmatory tests for identification of Candida strains
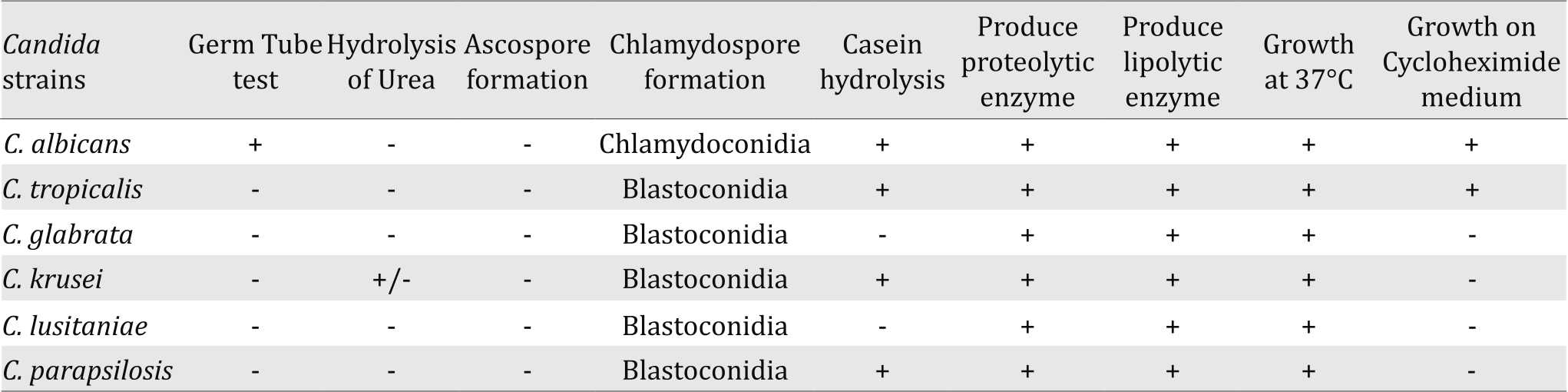
Germ tube test, hydrolysis of urea, Ascospore formation, and Chlamydospore formation along with assimilation and fermentation results confirmed the identification of the isolated Candida strains (Table 4).
Pathogenic potentialities of the isolated Candida strains
The pathogenic potentialities were tested for the isolated Candida strains and the results showed that the strains were able to grow at 37°C, which indicates their ability to grow at body temperature. Also, they could hydrolyze casein and patient’s fats indicating their ability to produce proteolytic and lipolytic enzymes (Table 4).
Table 4. Confirmatory tests for identification and pathogenic potentiates of Candida strains isolated from the study neonates in NICU
Overall, C. albicans was the most frequent spp. (58.8%) isolated from the neonates in NICU, followed by C. tropicalis (17.6%), and C. glabrata (15.6%). However, while C. albicans was the predominant early colonizing spp. (92.6%), the most common Candida spp. lately colonizing the neonate was C. tropicalis (33.3%) followed by C. glabrata (29.2%) then C. albicans (20.8%). The three Candida spp. isolated from hands of nurses were two C. glabrata and one C. tropicalis.
Of the 54 preterm neonates, 30 (55.5%) were colonized with Candida, which was more than colonization in full term neonates (7/18; 38.8%) and post term neonates (14/28; 50%). The most common Candida species isolated from the 30 colonized preterms was C. albicans (18; 60%) followed by C. tropicalis (7; 23.3%), C. glabrata (2; 6.7%), C. lusitamae (2; 6.7%), and C. krusei (1; 33.3%).
Neonatal risk factors related to early and late colonization as determined by univariate analysis are presented in Table 6. No risk factors was identified for early colonization, However, late colonization was significantly associated with BW <1.5 kg and total parenteral nutrition (Table 5).
Table 5. Neonatal risk factors for Candida colonization of the study neonates
Antifungal susceptibility testing
Candida albicans and C. tropicalis were found to be highly susceptible to azoles, especially to itraconazole in contrast to C. glabrata and C. krusei, which were totally resistant to azoles. Neonatal isolates of Candida species exhibited decreased susceptibility to amphotericin B where only 40% of C. albicans, 22% of C. tropicalis and none of C. glabrata and C. krusei isolates were sensitive to amphotericin (Table 6).
Table 6. Antifungal sensitivity of Candida species isolated from neonates in the NICU
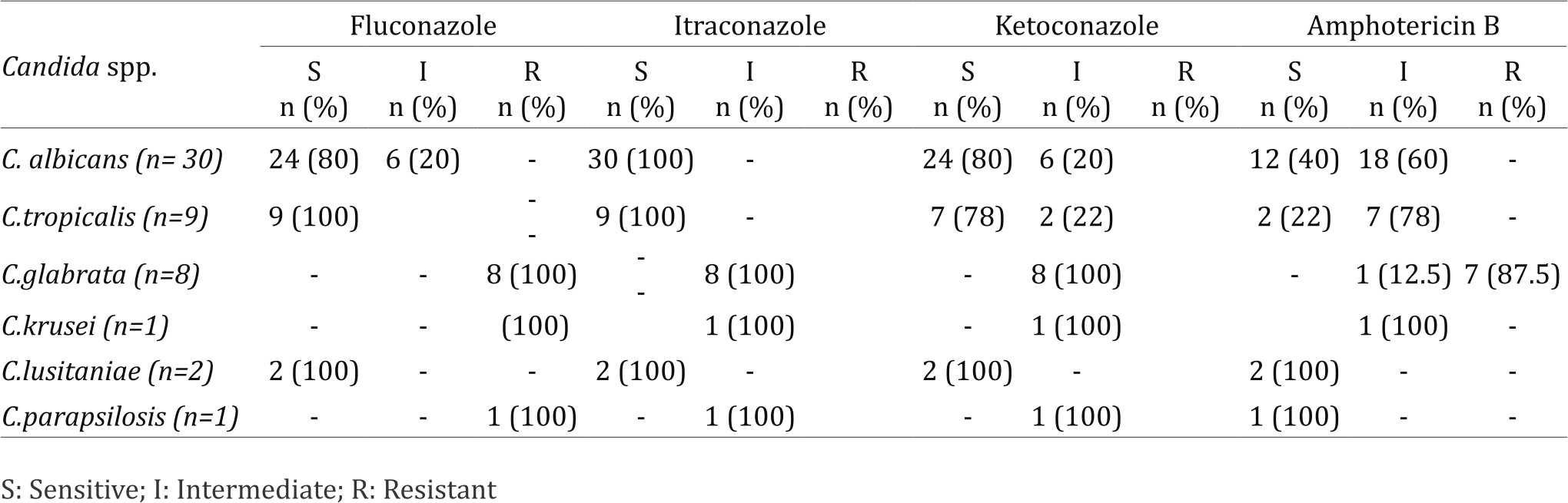
As regards to antifungal drug susceptibility of the 51 isolated Candida spp., 35 (68.6%) were sensitive to fluconazole, 41 (80%) to itraconazole and 33 (64.7%) to ketoconazole, while only 17 (33%) of Candida spp. were sensitive to amphotericin B.
As regards to the Candida species isolated from hands of nurses, C. tropicalis was sensitive to the azoles while the two C. glabrata were resistant to all antifungal drugs tested. These isolates gave the same pattern of susceptibility as that of the same spp. lately colonizing the neonates at the same week of sampling, suggesting a possible source of colonization.
DISCUSSION
Candida species generally colonize the skin, gastrointestinal tract, lower female genital tract, intertriginous areas (eg, groin and armpits), and the foreskin of the uncircumcised male. Similar to adults and older children, colonization usually precedes invasive fungal infection. Neonates are generally infected with a clone with which they had previously been colonized.11,15 In infants admitted to the NICU, colonization occurs in 30% to 60% of patients. Many studies have shown that the rate of colonization is dependent upon the birth weight of the infant. A retrospective analysis of weekly surveillance fungal cultures of the skin, gastrointestinal tract, respiratory tract, and umbilicus performed in 50 extremely low birth weight (ELBW) infants (birth weight below 1,000 g) from birth to six weeks of age demonstrated colonization by a Candidal species in 62 percent of infants by six weeks of age.16 Approximately 85 percent of the colonization occurred in the first two weeks of life. The skin and gastrointestinal tract were the first sites colonized, followed by the respiratory tract. Colonization was inversely related to gestational age. In a study of VLBW (birth weight below 1,500 g) infants, approximately 27 percent of patients were colonized with a Candidal species, of which one-third developed mucocutaneous candidiasis and 8% invasive Candidal infection15. The level of colonization is an important factor in developing invasive disease. The greater the density of organisms, the more likely it is that the organism will penetrate the host epithelial barriers, spread to the underlying tissue, and be disseminated through the blood stream.
In the present study about half of the 100 neonates admitted to the NICU were colonized with Candida. Early colonization was found in 27 (27%) neonates whereas 24 (24%) neonates were lately colonized during their stay in NICU. In a similar study done in during the same period by Mohamed et al17 they found that 62.5% of patients have become colonized by fungi during their stay. Mahieu et al4 also reported a much lower rate of early Candida colonization where only 1.2% of neonates were colonized. This notable difference might be attributed to their cultures included umbilical, ear, groin and axillary swabs, without culturing the oral mucosa which is a main site of early colonization. In addition, variation in the rate of colonization depends upon management protocol and nature and intensity of routine antifungal antiseptics measures applied in a particular setup.5
Fifty-four percent of study neonates were colonized with Candida at one site, while 45% were colonized at more than one site. Although the clinical relevance of Candida colonization at one site is limited, the relationship between multisite colonization and subsequent development of candidemia has been demonstrated by several investigators.18,19
Perianal area and oral cavity were the most frequently colonized sites (60% and 56%, respectively). This high rate of early gastrointestinal colonization is in accordance with observations of others who found rates up to 90% when rectal culture was done at birth.1,16 Gastrointestinal tract can serve as a reservoir from where the fungus can spread, particularly if there is a breach in mucosal lining20. The critical role of such colonization was confirmed by Saiman et al10 where GIT colonization was a risk factor for candidemia and molecular typing revealed that a high proportion of neonates had preceeding Candida colonization with the same clone that caused candidemia.
Overall, C. albicuns was the most frequent species (58.8%) isolated from the study neonates followed by C. tropicalis (17.6%), C. glabrata (15.6%), C. lusitaniae (4%), and C. kmsei and C. parapsilosis (2% each). In concordance with our findings is the study of Farmaki et al6 where the most frequent isolates were C. albicans (42%), C. tropicalis (24%), C. kmsei (11%), C. parapsilosis (7%), and C. glabrata (7%) while C. htsitanitie was isolated in one neonate in association with C. tropicalis.
In early colonization, C. albicans was the most common spp. (92.6%), which agrees with that reported by Mahieu et al4, where C. albicans was also the main colonizing spp. at birth (82%). On the other hand, the non-C. albicuns predominated in late colonization; C. tropicalis (33.3%), and C. glabrata (29.2%) while C. albicans represented 20.8%. In line with our findings, Mohamed et al17 reported that the non- C. albicans were the most commonly acquired fungi during the stay in PICU; C. glabrata (14.3%), C.tropicalis, (12.5), and C. krusei (7.1%) vs C. albicans ( 8.9%). An increase in the frequency of the non-albicans spp. isolated from neonates with late colonization was also demonstrated by Farmaki et al representing 59%.6
In accordance with our study, other investigators reported that most of the neonates who exhibit early colonization are colonized by C. albicans, presumably as being the most frequent species that colonizes the maternal vagina.4,6,21 In contrast, due to close relation of non- C. albicans species with NICU practices and materials, these strains more frequently colonize and infect neonates staying in the NICU for longer periods where horizontal transmission takes place.6
The increase in the non- C. albicans in several NICUs might also be attributed to the possibility of selection of less susceptible species by the extensive use of antifungal prophylaxis. Furthermore, the increasing use of several diagnostic and therapeutic interventions such as central venous catheter and parenteral nutrition might explain the shift away from C. albicans towards other species.22
The isolation of C. tropicalis in the present study is noteworthy, being more virulent than C. albicans and disseminated infection is associated with high mortality rates.23 Likewise is the isolation of C. glabrata which has been reported as a significant nosocomial pathogen in pediatric patients causing high mortality due to the development of a secondary resistance to fluconazole.10 Although only one of each of C. krusei and C. parapsilosis was isolated, yet it also must be noted too. C. krusei has been increasingly incriminated in serious disseminated infections while C. parapsilosis is considered a relevant nosocomial pathogen and the most frequent Candida colonizing catheters owing to its ability of biofilm formation.24
The above data show that preterm neonates colonization (55.5%) was more than in full term neonates (38.8%) and post term neonates (50%) and C. albicans was the most common colonizing species (60%), followed by C. tropicalis (23%). In the study of Mendiratta et al,5 colonization in preterm (33.9%) was significantly higher than in term neonates (10%) and C. albicans was also the main isolate (45.9%) followed by C. tropicalis (21.6%). Manzoni et al3 also reported C. albicans as the most frequent isolated spp. (83.5%), followed by C. parapsilosis (15.9%) and C. glabrata (7.9%). Increased colonization in preterm has been attributed to relative immunodeficiency such as decreased function of neutrophils and relative quantitative deficiency of maternal IgG to Candida.25
No risk factors in the present study were associated with early colonization. In the study of Farmaki et al6 and Mahieu et al,4 vaginal delivery was identified as a risk factor for early colonization and most likely represents acquisition during delivery. Its in line with what was stated by Saiman et al10 that delivery via cesarean section (CS) was protective from Candida colonization at birth. However, the association could not be detected in this study probably because more than 80% were born by CS.
Lower gestational age at birth was a significant risk factor for early neonatal colonization in the study of Mahieu et al.4 Although, not considered as risk factor in the present study, early colonization was more associated with preterms. The increased colonization in neonates of low gestational age can be explained by vulnerability of this group to critical illness that subject them to more invasive devices in NICU and aggressive antibiotic use predisposing them to fungal colonization.
Low BW and total parenteral nutrition administration were identified as risk factors for late colonization in the present study. LBW was also demonstrated by Saiman el al10 as a risk factor for Candida colonization and BW <1,500g was the only independent risk factor for late colonization in the study of Farmaki et al.6 The longer stay of low BW neonates in the NICU might explain why they are colonized by Candida spp. more frequently than larger neonates. In addition, degree of prematurity, central venous catheters and other factors could all be contributory.6 Total parenteral nutrition was significantly associated with Candida colonization. Saiman et al postulated that loss of normal GIT flora due to delayed enteral feeding may facilitate Candida species colonization.10
Antibiotics, especially third generation cephalosporin administration were found to be associated with colonization in neonates in many studies.6,10,11 Antibiotics are known to suppress immune system25 and administration to already compromised neonates promotes colonization,5 in addition to inhibition of the endogenous microflora and unopposed flourishing of Candida.26 However, an association could not be detected in the present study, as 89% of the study neonates have received third generation cephalosporin on admission.
Of the isolated Candida species, 68.6% were sensitive to fluconazole, 80% to itraconazole and 64.7% to ketoconazole, while only 33% were sensitive to amphotericin B. Candida isolates from NICU and PICU in the study of Kuzucu et al12 showed a higher susceptibility pattern where 93% and 82% were susceptible to fluconazole and itraconazole, respectively, while all were sensitive to amphotericin B. These results come in line with the study of Farmaki el al6 in NICUs, where C. albicans was highly susceptible to azoles. Kusucu et al12 reported that the sensitivity of C. albicans isolated from patients in NICU and PICU was 86.7% and 73.4% to fluconazole and itraconazole, respectively.
About third of Candida species isolated in the present study were sensitive to amphotericin B which is inconsistent with the findings of Farmaki et al6 and Kuzucu et al12 where Candida isolates from NICU and PICU patients remained completely susceptible to amphotericin B. Only 40% of C. albicans and 22% of C. tropicalis isolated in the present study were sensitive to amphotericin B.
No Candida was isolated from the environmental surfaces in NICU, however from the hands of HCWs, one C. tropicalis and two C. glabrata were isolated. These isolates had the same antifungal susceptibility pattern as the same species lately colonizing the neonates at the same time of sampling, indicating a probable source of colonization. In the study of Saiman et al10 29% of the HCWs hands were positive for Candida, where C. parapsilosis was cultured from 19%, C. albicans from 5%, while C. tropicalis was cultured from <1%.
Huang et al11 reported that acquisition of C. tropicalis very likely occurred in the NICU by cross-contamination. This was also suggested by Roilides et al27 in a molecular epidemiologic study of an outbreak of infection caused by C. tropicalis in NICU. Mendiratta et al5 reported no samples from NICU or hands of HCW were positive for Candida. Although, the examination of the hands of HCWs during the study period failed to document a common source of any Candida species, Farmaki et al6 could not exclude transient hand carriage of personnel. They suggested nosocomial acquisition of Candida predominantly non-albicans Candida, as a larger percentage (14.2%) of neonates were colonized at late stage during their NICU stay than at an early stage (2.5%).
As horizontal transmission from colonized hands of HCWs or, less commonly, from contaminated or surfaces, has been reported especially in the sicker neonates who require more handling by HCWs.10 Thus the importance of hand washing and compliance with guidelines for preventing nosocomial transmission must be emphasized in NICUs.
In conclusion, Candida has emerged as a common cause of infections in infants admitted to NICU, particularly preterm infants. Neonatal infections caused by non-albicans species occur at a later age during their stay in NICU and are more likely to be acquired from HCWs.
Most Candidal infections are due to vertical transmission from the mother. Candida generally colonizes the skin, gastrointestinal tract, lower female genital tract, intertriginous areas, and the foreskin of uncircumcised males. The rate of colonization increases with decreasing birth weight. Infants admitted to the NICU, especially premature infants, are at increased risk for Candidal infections
Conflicts of Interest
The author confirms no conflict of interest in this study.
Acknowledgment
The author acknowledges the help from technicians and supporting staff from the hospital at Riyadh and the institute where research was conducted.
REFERENCES
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2Pt1):285–91.
- Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics. 2006;117(5):1680–7.
- Manzoni P, Farina D, Leonessa M, d’Oulx EA, Galletto P, Mostert M, et al. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics. 2006;118(6):2359–64.
- Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. 2010;11(2):240–5.
- Mendiratta DK, Rawat V, Thamke D, Chaturvedi P, Chhabra S, Narang P. Candida colonization in preterm babies admitted to neonatal intensive care unit in the rural setting. Indian J Med Microbiol. 2006;24(4):263–7.
- Farmaki E, Evdoridou J, Pouliou T, Bibashi E, Panagopoulou P, Filioti J, et al. Fungal colonization in the neonatal intensive care unit: risk factors, drug susceptibility, and association with invasive fungal infections. Am J Perinatol. 2007;24(2):127–35.
- Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92(2):F127–9.
- Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol. 2003;27(5):357–64.
- Kaufman D. Fungal infection in the very low birthweight infant. Curr Opin Infect Dis. 2004;17(3):253–9.
- Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319–24.
- Huang YC, Li CC, Lin TY, Lien RI, Chou YH, Wu JL, et al. Association of fungal colonization and invasive disease in very low birth weight infants. Pediatr Infect Dis J. 1998;17(9):819–22.
- Kuzucu C, Durmaz R, Otlu B, Aktas E, Gulcan H, Cizmeci Z. Species distribution, antifungal susceptibility and clonal relatedness of Candida isolates from patients in neonatal and pediatric intensive care units at a medical center in Turkey. New Microbiol. 2008;31(3):401–8.
- Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345(23):1660–6.
- Clinical and Laboratory Standards Institute/ NCCLS. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A8. Wayne;PA: National Committee for Clinical Laboratory Standards; 2003.
- Sherertz RJ, Gledhill KS, Hampton KD, Pfaller MA, Givner LB, Abramson JS, et al. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. J Pediatr. 1992;120(3):455–61.
- Kaufman DA, Gurka MJ, Hazen KC, Boyle R, Robinson M, Grossman LB. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. Pediatr Infect Dis J. 2006;25(8):733–7.
- Mohamed SA, Ibrahim HM, Ahmed AE, El-Sayied SB. Pattern of fungal colonization in critically ill pediatric patients in Ain Shams Pediatric 1CU. Pediatric thesis, Egypt: Ain Shams University, Faculty of Medicine; 2009. Pp 35–67.
- Al-Tawfiq JA. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital, 1996-2004. Int J Infect Dis. 2007;11(3):239–44.
- Agvald-Ohman C, Klingspor L, Hjelmqvist H, Edlund C. Invasive candidiasis in long-term patients at a multidisciplinary intensive care unit: Candida colonization index, risk factors, treatment and outcome. Scand J Infect Dis. 2008;40(2):145–53.
- Segal, E. and Clad, D. Candida species and Blastoschizomyces capitus. In Topley and Wilson’s Microbiology and Microbial infections. 9th ed. Medical Mycology: London; 1998, p. 423–60.
- Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351(9):876–83.
- Resende JC, de Resende MA, Saliba JL. Prevalence of Candida spp. in hospitalized patients and their risk factors. Mycoses. 2002;45(8):306–12.
- Krcmery V, Barnes AJ. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J Hosp Infect. 2002;50(4):243–60.
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–94.
- Baley JE. Neonatal candidiasis: the current challenge. Clin Perinatol. 1991;18(2):263–80.
- Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Semin Perinatol. 2003;27(5):357–64.
- Roilides E, Farmaki E, Evdoridou J, Francesconi A, Kasai M, Filioti J, et al. Candida tropicalis in a neonatal intensive care unit: epidemiologic and molecular analysis of an outbreak of infection with an uncommon neonatal pathogen. J Clin Microbiol. 2003;41(2):735–41.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id