
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Antiproliferative activity and caspase enhancement properties of Annona muricata leaves extract against colorectal cancer cells
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i3.1449 Med J Indones. 2016;25:136–42
Received: May 17, 2016
Accepted: June 06, 2016
Author affiliation:
1 Department of Pharmacology, Faculty of Medicine, Christian University of Indonesia, Jakarta, Indonesia
2 Department of Nutrition, Faculty of Medicine, Universitas Indonesia, SEAMEO RECFON, Jakarta, Indonesia
3 Department of Pharmacology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Microbiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
5 Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
6 Department of Food Technology, Faculty of Engineering, Bina Nusantara University, Jakarta, Indonesia
7 Department of Pharmaceutical Biology, Faculty of Pharmacy, Gadjah Mada University, Yogyakarta, Indonesia
Corresponding author:
Lili Indrawati
E-mail: lili_zain@yahoo.com
Background
The prevalence of colorectal cancer is rising in Asia including Indonesia. Annona muricata tea leaves, that is traditionally used for maintaining health, and lately being used by cancer patients. The objectives of this study is to investigate its effects in human colorectal cancer cell in vitro and ex vivo.
Methods
Thirty patients with colorectal cancer (CRC) were enrolled in a randomized double-blind placebo-controlled trial. They were equally divided into two groups: those treated with 300 mg A. muricata leaf extract and placebo daily for 8 weeks. Serum from supplemented CRC patients of both groups was compared for caspase 9 and caspase 8 enhancement activity. Antiproliferative effect of water extract of A. muricata leaves and its fractions were evaluated against colorectal cancer cell line (DLD-1 and COLO 205) compared with 5-fluorouracil and placebo, the dose range was 62.5- 2,000 μg/mL. Method used was 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data were analyzed by Mann-Whitney U test. The p value was set at 0.05.
Results
Ethanol-soluble fraction of A. muricata leaves extract water extract (ESFAM) leaves extract had cytotoxicity effects on DLD-1 as well as COLO 205 cell line, as shown by the lower IC50 compared to 5-fluorouracil and placebo, 20.59 μg/mL and 654.9μg/mL, respectively. Serum of subjects supplemented with extract significantly induced caspase 9 (p=0.001) activity of DLD-1 colorectal cancer cell line, but not for caspase 8 activity (p=0.372).
Conclusion
The study's results suggest the cytotoxicity potential of A. muricata leaves extract in in vitro and ex vivo studies.
Keywords
A. muricata leaves, cytotoxicity, colorectal cancer cell line, ex vivo, in vitro
The prevalence of colorectal cancer is rising in Asia1 and it is now the third most common malignant disease in both men and women. There are two to four folds increase of cancer incidence in the last five years.2 In Indonesia colorectal cancer was recently estimated to be the five most prevalent cancer found in males and females among 13 cancer registries.3
Many types of cancer have the mechanism to avoid apoptosis induced by anticancer drugs.4 The intervention of multistage carcinogenesis by regulating intracellular signaling pathways may give molecular basis of chemoprevention with a wide variety of dietary phytochemicals.5 Therefore, there is a growing interest to explore the possibility of using phytochemicals such as the ones found in our diet as chemopreventive agents.
Members of family Annonaceae have been investigated as potential sources of biologically active Annonaceous acetogenins, some of which has demonstrated a powerful anti-tumor activities.6 Currently, 34 acetogenins have been identified in the leaves of Annona muricata Linn.7 People have used A. muricata leaves traditionally by brewing in hot water and this preparation is found to be safe.8 The cytotoxicity of acetogenins has been shown to be stronger in tumorous than in normal cells.9 The primary site of action of the acetogenins is complex I of the electron transport chain in mitochondria.10 Study in mice showed that annonacin inhibited the normal growth of lung tumors during two-weeks period.11
Phytochemical assessment of A. muricata leaves showed the presence of alkaloids, tannins, flavonoids, saponins, anthraquinones and cardiac glycosides, ellagic acid, polyphenolic compounds, triterpenoids, β-sistosterol.12-14 Polyphenols such as flavonoid quercetin and flavone (2-phenyl-4H-1-benzopyran-4-one) may have chemoprevention property by reducing the incidence of many types of cancers, especially colon cancer.4
Caspase-3 activity and deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assessment showed that the ethanolic extract of A. muricata induced apoptosis in the myelogenous leukemic K562 cell line. A. muricata is considered to be a potential candidate for the development of pro-apoptotic drugs.14 Based on the findings, it is interesting to study the effect of A. muricata leaves extract towards cell proliferation as well as towards caspase-8 and caspase-9 in colorectal cancer cell in vitro. This study was the first ex vivo experiment using serum of colorectal cancer (CRC) patients after eight weeks treatment with the plant extract.
METHODS
The subjects for the ex vivo trial were CRC patients receiving outpatient care at the Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, after primary tumor resection. The protocol of this study was approved by Medical Ethics Committee, Faculty of Medicine, Universitas Indonesia (No. 406/H2.F1/ETIK/2013). Participation in the study was voluntary, and written informed consent was obtained prior to the study. The clinical trial is registered on ClinicalTrials.gov under the identifier NCT02439580.
Male and female CRC patients older than 30 years who had undergone primary tumor resection and were willing to take one capsule per day of A. muricata extract or a placebo as an additional treatment throughout the study period were included in the study. In addition, the patients were required to have satisfactory hematological and biochemical function and Karnofsky performance status of ≥60%.
Patients with the following conditions were excluded from the study: uncontrolled hypertension (untreated systolic blood pressure >160 mm Hg, or diastolic blood pressure >95 mm Hg); serious heart problems; upper limit of serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and creatinine were 111 U/L, 123 U/L, 3.6 mg/dL respectively; a disability rendering them unable to communicate verbally; or a history of cancers other than colorectal (such as non-melanoma skin cancer, basal cell carcinoma, and squamous cell carcinoma) in the past five years. Pregnant or lactating women, and those not using adequate contraception, were also excluded. In addition, patients taking other investigational drugs, patients with hereditary non-polyposis colorectal cancer (HNPCC), and patients taking probiotic supplementation during the study period were also excluded to avoid potentially conflicting conditions and treatments.
Annona muricata L. extract
The A. muricata extract used in this study is a standardized vacuum dried extract (Zirzak Orac) produced by Javaplant, Central Java, Indonesia, containing 0.018% acetogenin (w/w). Zirzak Orac underwent further fractionation using ethanol to produce ethanol-soluble fraction of A. muricata leaves water extract (ESFAM). ESFAM contains 0.36% acetogenin (w/w) or 3.6 mg/g, and a 10 g water extract is equivalent to a 2 g ethanolic fraction.
In this study, the CRC patients consumed either 300 mg of ESFAM or maltose as a placebo in the form of a capsule after breakfast.
Procedures
A randomized double-blind placebo-controlled trial (RCT) was conducted. The patients were randomly assigned into either ESFAM or placebo, through block randomization (four patients per block), supplementation was administered for eight weeks.
Peripheral blood samples were drawn from the patients through vein at the baseline and at the end of the study period. Venous blood samples used for the ex vivo study were centrifuged at 3,000 rpm for 10 minutes to obtain serum and then labeled and maintained at -80°C until analysis. ex vivo study was performed by treating colorectal cell lines blindly with the serum of patients from both groups.
Best result of ex vivo study could be achieved by using treated patients cancer cells. Before using commercial cell lines from American Type Culture Collection (ATCC®), efforts to develop cell line derived from CRC patients were failed as the cells from patients underwent surgery was highly contaminated since these are colorectal tissues.
The human colorectal cell line types used in this study were COLO 205 and DLD-1 and were purchased from ATCC® (Catalog No. CCL-222 and Catalog No. CCL-221, respectively); they were maintained according to supplier guidelines (American Type Culture Collection, ATCC, Manassas, VA). DLD-1 is derived from the Dukes’ type C human colorectal adenocarcinoma tissue, and COLO 205 is derived from the Dukes’ type D human colorectal adenocarcinoma tissue. Both lines were isolated from metastatic sites but there are differences, DLD-1 is from local metastatic site (lymph node) while COLO 205 is from a distant site (ascites). Both types of cell lines were chosen to represent both type of metastases.
Cells were incubated with 95% air and 5% CO2 at 37°C; all cells were maintained below passage 20 and used in experiments during the linear phase of growth. The human colorectal cancer cell lines DLD-1 and COLO 205 were maintained in RPMI-1640 medium that supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin and maintained in a humidified environment containing 5% CO2 at 37°C.
in vitro cytotoxic test by MTT assay
Antiproliferative effect of water extract of A. muricata leaves and its fractions, dose range 62.5–2,000 μg/mL, were evaluated against colorectal cancer cell line DLD-1 and COLO 205 with micro-culture assay based on the metabolic reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT); compared with 5-fluorouracil (5-FU) and placebo.
The cytotoxic activity of extract was assessed using an MTT assay; cells were cultured in 96- well micro titer plates, where each well contained 2×104 cells, and treated for 48 hours. Cytotoxicity was assessed using the MTT test (Trevigen’s TACS® MTT cell proliferation assay), in triplicate.15-16
Caspase activity assessment
Since apoptosis might be one of the prime candidate mechanisms, we analyzed the expression level of caspase-8 and caspase-9. The DLD-1 colorectal carcinoma cell line was chosen since it was shown to be a proper in vitro model as previously described.17 Cleaved caspase-8 was measured using kit from R&D Systems, Inc. (USA & Canada) Catalog No. KCB705. This cellbased enzyme-linked immunosorbent assay (ELISA) contains the components required to run an ELISA using fluorogenic substrates to measure cleaved caspase-8 (Asp391) in whole cells.
This simple and efficient assay removes the need to prepare cell lysates and can be used to investigate signaling pathways and the inhibitory property on cells. Cells were fixed and permeabilized in the wells after being grown in 96-well microplates and stimulated with ligands.
The target protein amount was assessed using a double immune enzymatic labeling procedure. Two primary antibodies were used to simultaneously incubate the cells. They are an antibody specific for the target protein and a normalization antibody that is specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping protein. The primary antibodies are obtained from different species. Two secondary antibodies recognizing the different species are labeled with either horseradish-peroxidase (HRP) or alkaline phosphatase (AP), and two spectrally distinct fluorogenic substrates for either HRP or AP are employed for detection. The fluorescence of the target protein is normalized to that of the total GAPDH in each well for the correction of well-to-well variations.
This two-wave length assay results in precise analysis of cleaved caspase-8 (Asp391) with good reproducibility. Cleaved caspase-9 was measured using kit from eBioscience company, Catalog No. BMS2025/BMS2025TEN (USA & Canada).
Concentrations of extracts used to test the caspase activity are 25–50 μg/mL, as compared to 25–50 μg/mL 5-FU as positive control. The extracts data were complemented by parallel studies of direct addition of participants’ serum to the same human cell lines.
ex vivo study was performed by treating colorectal cell lines with serum of patients from both groups; treated with leaves extract or placebo. Sera from patients of both groups were compared for caspase-8 and caspase-9 enhancement activity.
Statistical analysis
SPSS for Windows version 22 (SPSS Inc., USA) was used to analyze the results. The Saphiro Wilk test was used to test the normality of the data. Independent T tests was used to examine differences in means for normally distributed data, otherwise Mann Whitney U test was used. The p value was set at 0.05.
RESULTS
Cytotoxicity
Inhibitory concentration (IC)50 values of the different extracts against DLD-1 are shown in Figure 1. ESFAM showed the lowest IC50 values, 20.59 μg/mL.
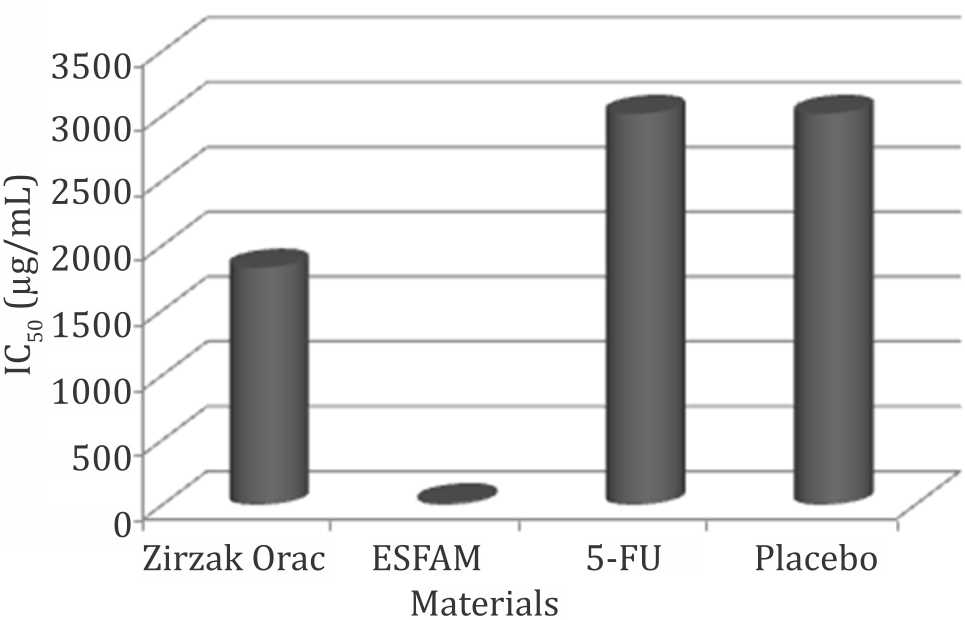
Figure 1. IC50 of materials against DLD-1. ESFAM: ethanolsoluble fraction of A. muricata water extract; 5-FU: 5-fluoro uracil
Zirzak Orac and ESFAM showed the lower IC50 values against COLO 205 compared to 5-FU which were 277.7 μg/mL and 654.9 μg/mL, respectively (Figure 2).
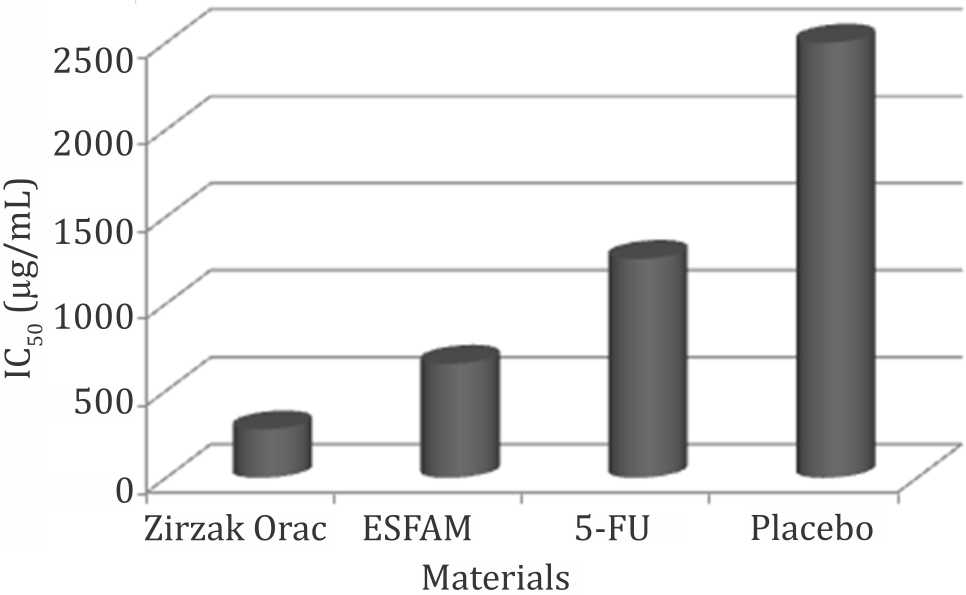
Figure 2. IC50 of materials against COLO 205. ESFAM: ethanol-soluble fraction of A. muricata water extract; 5-FU: 5-fluorouracil
Caspase activity
The activity of caspase-8 and caspase-9 of DLD- 1 colorectal cancer cell line tended to be higher when treated with the extracts as compared to 5-FU as positive control, as well as untreated cell and blank that only contained medium (Figure 3).
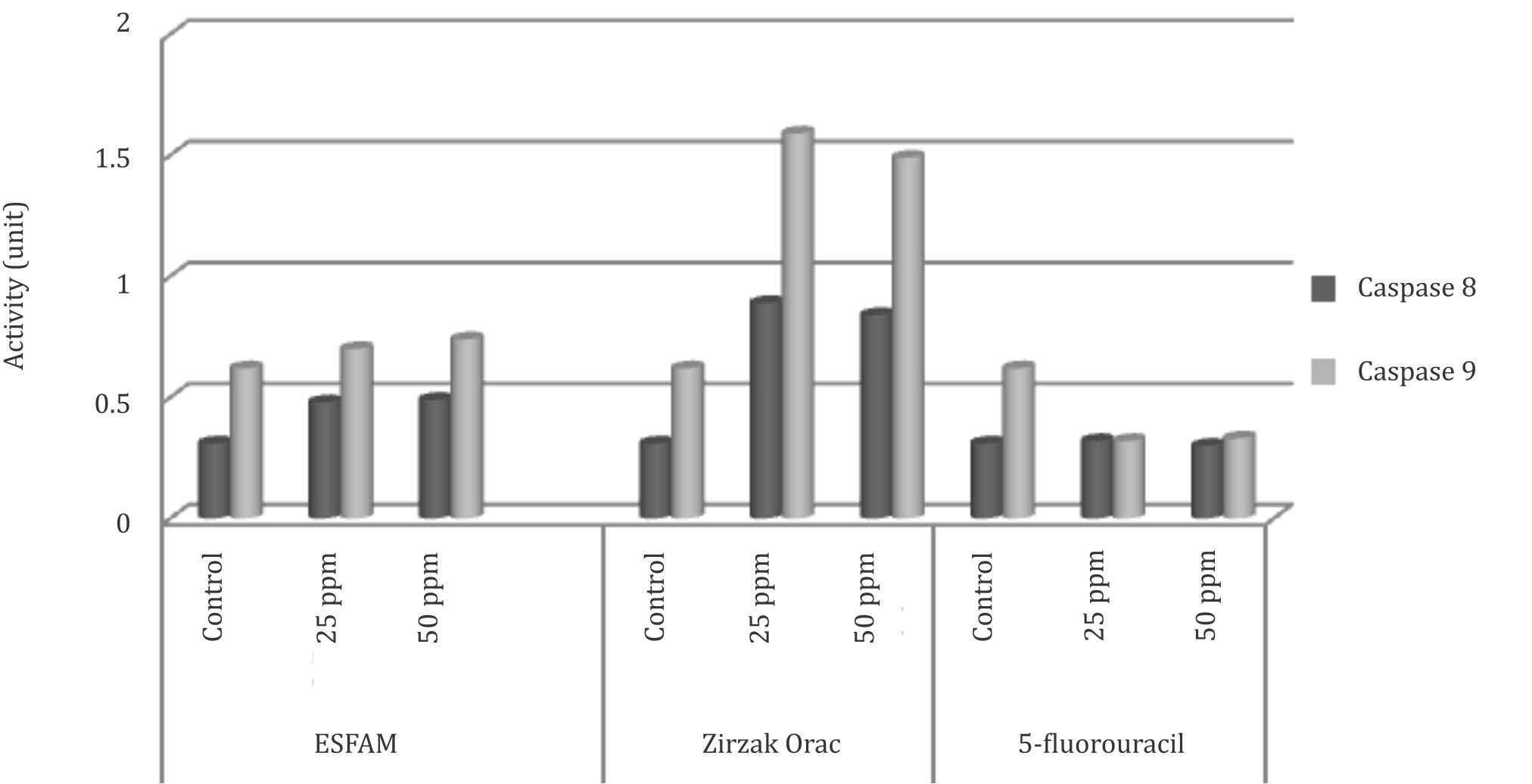
Figure 3. Caspase-8 and caspase-9 activities of DLD-1 colorectal cancer cell lines in vitro. ESFAM: ethanol-soluble fraction of A. muricata water extract
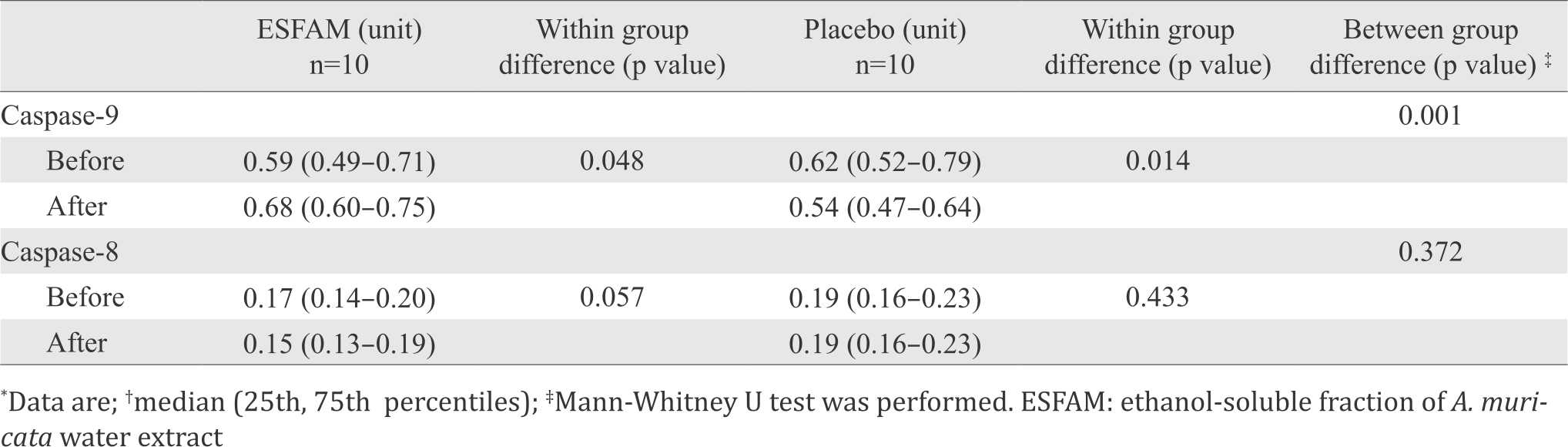
ex vivo experiment on caspases activities were conducted using subjects’ serum to stimulate caspase-8 and caspase-9 activities of DLD-1 colorectal cell lines. The results showed that serum from patients treated with ESFAM significantly enhanced the expression of caspase-9 (p<0.05) compared to placebo group. Serum from placebo group has significantly lower expression of caspase-9. Expression of caspase-8 tended to decrease in both groups (p=0.057) (Table 1).
Table 1. Caspase-8 and caspase-9 activities of DLD-1 colorectal cell lines ex vivo before and after treatment
DISCUSSION
The cytotoxic activity of different extracts of A. muricata leaves have been assessed against human colorectal cancer cell lines COLO 205 and DLD-1. There was a dose dependent manner in decreasing viable cells after treatment with ESFAM and Zirzak Orac (0.0625–2 mg/ml) for 48 hours both in COLO 205 and DLD-1 cell lines. ESFAM showed the lowest IC50 against DLD-1, while Zirzak Orac and ESFAM had the lowest IC50 against COLO 205.
These findings are consistent with previous in vitro studies in demonstrating the cytotoxic activity of the leaves extracts, as well as its fractions, against colon adenocarcinoma (HT- 29) cell lines, pancreatic carcinoma cell (PACA-2), prostate adenocarcinoma (PC-3), hepatoma cell lines Hep G2 clone 2,2,15 and HeLa cell culture and T47D breast cancer cell lines.18-19 Ethanolic extract of the leaves from Colombia, Indonesia, and Taiwan showed inhibitory activities against Cells-MDBK, CA-Mammary-MCF-7, and HepG2 clone 2,2,15 respectively. Ethanolic extract of the leaves from Borneo, Costa Rica, USA showed inhibitory activities against cell culture CA-9KB.11 In vivo study in mice showed that annonacin inhibited the growth of the lung tumors during two-weeks period, it did not eradicate the tumors nor stop their growth in mice.
This study revealed the superiority of the extracts in vitro against colon cancer cells compared to 5-FU; and consistent with other studies showed that acetogenins as well as the extract are more potent than the standard drugs (adriamycin, vincristine, and vinblastine).10-11 Data from previous study on colon cancer cell lines suggested that there were pathways for 5-FU resistance including altered regulation of nucleotide metabolism, amino acid metabolism, cytoskeleton organization, transport, and oxygen metabolism.20 The cytotoxicity of acetogenins has been shown to be stronger in tumorous than in normal cells.9 This study showed that ESFAM with the higher concentration of annonacin showed similar cytotoxicity against both types of cell lines.
Study on caspase activity was conducted to determine the mechanisms of action of the cytotoxicity of A. muricata leaves extracts. Caspase-8 and caspase-9 activities were tested both in vitro and ex vivo on human colorectal cancer DLD-1 cell lines. Caspase-821 and caspase-917 were the most important regulator in DLD-1.
The activities of both caspases tend to increase by induction of ESFAM and Zirzak Orac compared to 5-FU and control cell in vitro. These results are in agreement with previous studies, where ethanolic extract of A. muricata induced apoptosis in the myelogenous leukemic K562 cell line through inducing caspase-3 activity and deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL).22
This study was the first ex vivo study for A. muricata leaves extract that exposing CRC cell lines with patients serum after eight weeks treatment with ESFAM. The use of serum from patients supplemented with ESFAM was aimed to overcome the limitation in conducting bioavailability study, which was not currently possible due to uncertainty of bioactive compounds in the extract. in vitro study on ESFAM may reflect the effect of the extract against the cell tumor directly when it passed through the tumor in colon, and ex vivo study using patients’ sera represented the effect of ESFAM after being absorbed and entered the blood circulation.
The result of ex vivo caspase activity study is consistent with the in vitro study. Patients’ serum from ESFAM group significantly increased the activity of caspase-9 compared to placebo group. On the contrary, patients’ serum from placebo group tended to decrease caspase-9 activity.
Annonaceous acetogenin has been shown previously inducing apoptosis in cancer cell lines by involving multiple pathways. The primary site of action of the acetogenins is complex I of the electron transport chain in mitochondria.10 Acetogenins have also been shown being the potent inhibitors of NADH oxidase in plasma membranes of cancerous liver cells. Specifically, the bis-THF acetogenin bullatacin was shown to be a strong inhibitor of this enzyme in liver cancer cell; however, bullatacin showed no inhibition of the NADH oxidase against “normalized” liver cells.23
The enhancement of caspase-9 activity in this study is consistent with the above studies where caspase-9 plays key role in intrinsic pathway, namely mitochondrial-mediated pathway. Patients’ serum induces cytotoxicity after consumption of A. muricata leaves extract most probably mediated through the effect of the extract in enhancing caspase-9 activity.
Activity of caspase-8 in ex vivo study was contradictory with result of in vitro study, where caspase-8 activity tended to decrease instead of increase. This result may indicate that the pathway for apoptosis capacity is not through extrinsic pathway. The non-significant result may also due to inadequate dose given to subjects, resulted in an insufficient amount in the serum to enhance the caspase-8 activity. It may also due to low concentration of serum in the cell culture media, which is only 10%. Number of caspase-8 reseptor on DLD-1 cell lines may also contribute to this result.
In conclusion, A. muricata leaves water extract and its ESFAM exhibited cytotoxic activities in colorectal cancer cells in vitro. ESFAM significantly induced caspase-9 activity of DLD-1 colorectal cancer cell line ex vivo using serum of colorectal cancer patients after eight weeks treatment with ESFAM.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The authors are grateful to the Centre for Ageing Studies, Universitas Indonesia for funding this study, contract No.036/H2.R12.5/μG/ ML.01.04/2012. The authors thank Sofy Meilany for the laboratory work and Rina Agustina for fruitful discussion.
REFERENCES
- Yee YK, Tan VP, Chan P, Hung IF, Pang R, Wong BC. Epidemiology of colorectal cancer in Asia. J Gastroenterol Hepatol. 2009;24(12):1810–6.
- Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012;4(4):68–70.
- Soeripto, Indrawati, Indrayani. Gastro-intestinal cancer in Indonesia. Asian Pac J Cancer Prev. 2003;4(4):289–96.
- Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007;6(9):2591–9.
- Khan AQ, Bury JP, Brown SR, Riley SA, Corfe BM. Keratin 8 expression in colon cancer associates with low faecal butyrate levels. BMC gastroenterol. 2011;11(1):2–10.
- Yu DQ. Recent works on anti-tumor constituent from Annonaceae plants in China. Pure Appl Chem.1999;71(6):1119–22.
- Champy P, Melot A, Guérineau Eng V, Gleye C, Fall D, Höglinger GU, et al. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in guadeloupe. Mov Disord. 2005;20(12):1629–33.
- Zuhud E. Kanker Lenyap Berkat Sirsak. 1st ed. Indah Y, editor. Jakarta: PT AgroMedia Pustaka; 2011. p. 37–39. Indonesian.
- García-Aguirre KK, Zepeda-Vallejo LG, Ramón-Gallegos E, Alvárez-González I, Madrigal-Bujaidar E. Genotoxic and cytotoxic effects produced by acetogenins obtained from Annona cherimolia Mill. Biol Pharm Bull. 2008;31(12):2346–9.
- Gupta A, Pandey S, Shah D, Yadav J, Seth N. Annonaceous acetogenins: the unrevealed area for cytotoxic and pesticidal activities. Syst Rev Pharm. 2011;2(2):104–9.
- Taylor L. Technical data report for graviola Annona muricata. In: Taylor L, editor. Herbal Secretes of the Rainforest. Austin: Rain Tree; 2002. p. 1–43.
- Marino DC, Sabino LZ, Armando J Jr, Ruggiero Ade A, Moya HD. Analysis of the polyphenols content in medicinal plants based on the reduction of Cu(II)/bicinchoninic complexes. J Agric Food Chem. 2009;57(23):11061–6.
- Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2008;6(1):30–41.
- Ezirim AU, Okachi VI, James AB, Adebeshi OA, Ogunnowo S, Odeghe O. Induction of apoptosis in myelogenous leukemic K562 cells by ethanolic leaf extract of Annona muricata. Global J Res Med Plants & Indigen Med. 2013;2(3):142–51.
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48(3):589–601.
- Vistica DT, Skehan P, Scudiero D, Monks A, Pittman A, Boyd MR. Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res. 1991;51(10):2515–20.
- Schmid J, Dussmann H, Boukes GJ, Flanagan L, Lindner AU, O’Connor CL, et al. Systems analysis of cancer cell heterogeneity in caspase-dependent apoptosis subsequent to mitochondrial outer membrane permeabilization. J Biol Chem. 2012;287(49):41546–59.
- Kim GS, Zeng L, Alali F, Rogers LL, Wu FE, McLaughlin JL, et al. Two new mono-tetrahydrofuran ring acetogenins, annomuricin E and muricapentocin, from the leaves of Annona muricata. J Nat Prod. 1998;61(4):432–6.
- Chang FR, Liaw CC, Lin CY, Chou CJ, Chiu HF, Wu YC. New adjacent Bis-tetrahydrofuran Annonaceous acetogenins from Annona muricata. Planta Med. 2003;69(3):241–6.
- De Angelis PM, Svendsrud DH, Kravik KL, Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FUresistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20–44.
- Bartke T, Siegmund D, Peters N, Reichwein M, Henkler F, Scheurich P, et al. p53 upregulates cFLIP, inhibits transcription of NF-kappaB-regulated genes and induces caspase-8-independent cell death in DLD-1 cells. Oncogene. 2001;20(5):571–80.
- Dodge MW. Synthesis of annonaceous acetogenins via asymmetric double cycloetherification [dissertation]. United States: University of Wisconsin-Madison; 2008.
- N, T. (2017). Mau nanya dong dok. [online] Mau nanya dong dok. Available at: https:// nanyadongdok.blogspot.com [Accessed 2 Jul. 2017].
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id