
Section Abstract Introduction Methods Results Discussion Conflict Of Interest Acknowledgment References
Basic Medical Research
The effects of quercetin on oxidative stress and fibrosis markers in chronic kidney disease rat model
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i3.1462 Med J Indones. 2017;26:169–77
Received: May 30, 2016
Accepted: August 31, 2017
Author affiliation:
Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
Corresponding author:
Vivian Soetikno
E-mail: vivian_09st@yahoo.com
Background
Oxidative stress may play a role in the pathogenesis of (CKD), Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor involved in cell defense mechanism against oxidative stress. In this study, we examined the effect of quercetin, a polyphenplic antioxidant anti fibrosis compund in fruits and vegetables, on the 5/6 nephrectomy-induced CKD progression model rats through modulation of Nrf2 expression.
Methods
Male Sprague-Dawley rats were randomly divided into normal control group (C), untreated 5/6 nephrectomy (Nx), quercetin-treated 5/6 nephrectomy (100 mg/kgBW/ day orally) (NxQ), and captopril-treated 5/6 nephrectomy (10 mg/kgBW/day orally) (NxK) for 8 weeks. At the end of study, all animals were sacrified. Urine, blood, and kidney tissues were taken for examination of proteinuria, plasma creatinine, urea, malondialdehyde (MDA), glutathione peroxidase (GPx) activity, Nrf2, Keap1, heme oxygenase-1 (HO-1) expressions, and renal fibrosis.
Results
Quercetin administration did not affect the level of protein in urine, plasma creatinine, and urea. However, it tended to reduce the level of MDA, increase GPx activity, Nrf2, Keap1, and HO-1 expression as well as the degree of fibrosis.
Conclusion
In 5/6 nephrectomized rats, quercetin tended to ameliorate the level of MDA, GPx activity, Nrf2, Keap1, and HO-1 expression. In addition, quercetin tended to decrease the degree of fibrosis in the remnant kidney.
Keywords
chronic kidney disease, nephrectomy, Nrf2, oxidative stress, quercetin
Chronic kidney disease (CKD) is a serious public health problem characterized by a progressive and irreversible kidney damage resulting in a relentless decrease of renal function.1,–3 According to data from the Health Research Association in 2013, Indonesia has a 0.2% increase of CKD prevalence.4 It has been shown that oxidative stress and inflammation play an important role in the pathogenesis of CKD by a variety of mechanisms including the activation of nuclear factor-kappa B and the production of reactive oxygen species (ROS), reactive nitrogen species, halogen by a leukocytes, and resident cells.5 In addition to oxidative stress and inflammatory process, recent study showed that next to the changes in the expression of coagulation factors and plasma kallikrein, protein related to blood coagulation and platelet activation were also played important roles in the progression of CKD.6
Inhibition of progression of CKD is important. Several animal studies used active compounds that acted as antioxidants, anti-inflammatory, or antifibrosis to inhibit the progression of CKD. It improved the structure and the function of kidney through the reduction of oxidative stress and inflammation. We have showed that curcumin, a powerful antioxidant, can attenuate oxidative stress, inflammatory response, and renal fibrosis in rats with 5/6 nephrectomy via activation of nuclear factor eryhtroid 2-related factor 2 (Nrf2).7 Nrf2, which binded to its inhibitor Kelchlike ECH-associated protein 1 (Keap1), was a transcription factor responsible for the induction of cytoprotective and antioxidant enzymes such as heme-oxygenase-1 (HO-1) and glutathione peroxidase (GPx).8 Therefore, compounds that act as antioxidants or induce Nrf2 may have a potential to be used as a therapy in CKD.
Quercetin is a polyphenolic compound found in common vegetables and fruits.9,10 It has many benefits for health such as an antioxidant, antifibrotic, anticancer11,12 and anti-inflamamation.13 In vitro study suggests that quercetin could be used againts oxidative stress caused by dimethoate in human blood lymphocyte.14 Quercetin increased the level of Nrf2 and stimulated gene expression of NADPH Quinones Oxireductase mediated by Nrf2-ARE in human hepatoma HepG2 cells.15 Moreover, in vivo study suggests that quercetin ameliorated kidney injury in diabetic nephropathy.16
We hypothesized that quercetin might ameliorate the progression of CKD through inhibition of oxidative stress and fibrosis. To elucidate this issue, weanalyzed the effects of quercetin on the progression of CKD using 5/6 nephrctomy model through Nrf2 pathway, a transcription factor responsible for cellular defense and survival pathway against oxidative stress.
METHODS
Animals
Male Sprague Dawley rats (150–300 g) were obtained from the National Agency of Drug and Food, Republic of Indonesia. Animals were housed in constant temperature room, maintained in 12:12 light–dark cycle, and allowed free access to food and water. The experimental protocols were approved by the Health Research Ethics Committee, Faculty of Medicine, University of Indonesia (No. 190/H2.F1/ETIK/2014).
Experimental Protocol
Animals were randomly divided into four groups (n=6 each), namely the normal control (C) group which underwent sham operation, untreated 5/6 nephrectomy (Nx) group, quercetin-treated 5/6 nephrectomy (NxQ) group, and captopriltreated 5/6 nephrectomy (NxK) group. Untreated 5/6 nephrectomy (Nx) group underwent 5/6 nephrectomy by surgical resection in the ventral abdomen to expose the left kidney, place a piece of suture in the upper and lower thirds of the left kidney, and ligate around each pole of the kidney at its one-third position. The one-third kidney on each pole was excised beyond the ligatures. This procedure was followed by a whole right kidney ablation seven days later. Furthermore, quercetin-treated 5/6 nephrectomy (NxQ) group underwent 5/6 nephrectomy, and one week after the second surgery, they received 100 mg/kg oral quercetin daily for eight weeks. Captopril-treated 5/6 nephrectomy (NxK) group underwent 5/6 nephrectomy, and one week after the second surgery, they received 10 mg/kg oral captopril daily for eight weeks.
Quercetin and captopril were dissolved in 0.5% carboxymethyl cellulose. The surgical procedures were carried out under general anesthesia (Ketalar 50 mg/kg i.p.) using an aseptic technique and given ampicillin 25 mg/kg for three days for infection prophylaxis. During the study, the body weight was measured every week. Twenty fourhour urine samples collected in metabolic cages at baseline (t0), before oral therapy (t1), four week after oral therapy (t2), and 12 week after oral therapy (t3). At the end of study, the animals were decapitated. Blood samples and kidney tissues were collected for further analysis. We used 5/6 nephrectomy model as it mimicked the progressive chronic kidney disease after loss of renal mass in human. It was shown that the imbalance in redox status was evident at an early stage of CKD and became more profound with the progression of kidney diseases. Therefore, we used quercetin as a potent antioxidant to halt the progression of CKD in animal model.
Blood and urine chemistries
Blood samples from decapitation were collected in heparin tubes and centrifuged at 3000 rpm (10 min, 4°C) for separation of plasma. The collected plasma was utilized for determination of creatinine and urea. Plasma creatinine level was determined by Jaffe method whereas urea level by urease and glutamate dehydrogenase enzymatic reaction method. The collected 24-hour urine was centrifuged at 3000 g (10 min, 23°C). Volume and protein content was measured, and protein in urine was determined by Bradford method.
Measurement of malondialdehyde (MDA) level
Kidney tissues (100 mg) were rinsed and homogenized in 1 mL of 0.1 M phosphate buffer saline (pH 7.4). After centrifugation at 3000 rpm (10 min, 4°C), the supernatants were collected and analyzed by TBA colorimetric method. In addition, the level of MDA was measured spectrophotometrically on UV-VIS spectrophotometer at 535 nm.
Measurement of GPx activity
Kidney tissues (100 mg) were homogenized in 1 mL of 0.1 M phosphate buffer saline (pH 7.4) and added 0.1% protein inhibitor cocktail. After centrifugation at 3000 rpm (10 min, 4°C), the supernatants were collected and analyzed. GPx activity was measured using spectrophotometer according to assay kit instructions (Randox). The oxidation of reduced nicotinamide adenine dinucleotide phosphate (NADPH) to nicotinamide adenine dinucleotide phosphate was measured by the decrease in absorbance at 340 nm.
Immunohistochemistry of Nrf2
Formalin-fixed, paraffin-embedded kidney tissue sections were used for immunohistochemistry staining. After deparafinization and hydration, the slides were washed in PBST. Immunohistochemistry staining was performed using NovolinkTM Polymer Detection System with the primary antibody (Nrf2) purchased from Santa Cruz (diluted 1:50). The procedures of staining were based on assay kit instruction. Microphotography analysis was determined using microscope photo-optilab (Optilab® Image Raster v.2.1). The number of positive cells (stained brown in nucleus) was counted on five large fields randomly at x400 magnification.
Histopathological analysis
Light microscopy was performed in formalinfixed sections (4 μm) with Masson’s Trichrome staining as indicator of fibrosis. Fibrosis was graded by two independent pathologists. To analyze renal fibrosis, we used analysis criteria by Chen et al12 and Gibson-Corley et al.17 The accumulation of collagen in the interstitial renal cortex was up to 5%, and the renal capsule was not thick. The slightly collagen accumulation (6–25%) accompanied by a thickening of the renal capsule, moderate accumulation of collagen (26–50%) accompanied by a thickening of the renal capsule, and severe collagen accumulation (>50%) accompanied by a thickening of the kidney capsule (sometimes with glomerulosclerosis) were graded as 0, 1, 2 and 3 respectively.
RNA extraction
Total RNA was extracted after kidney homogenization using Ultra TurraxT8 in TriPure isolation reagent (Roche Life Sciences) according to the standard protocol. cDNA was synthesized by using total RNA (2 mg) as a template and was synthesized according to Transcriptor first strand cDNA synthesis kit (Roche Life Sciences).
Gene expression analysis by real-time RT-PCR
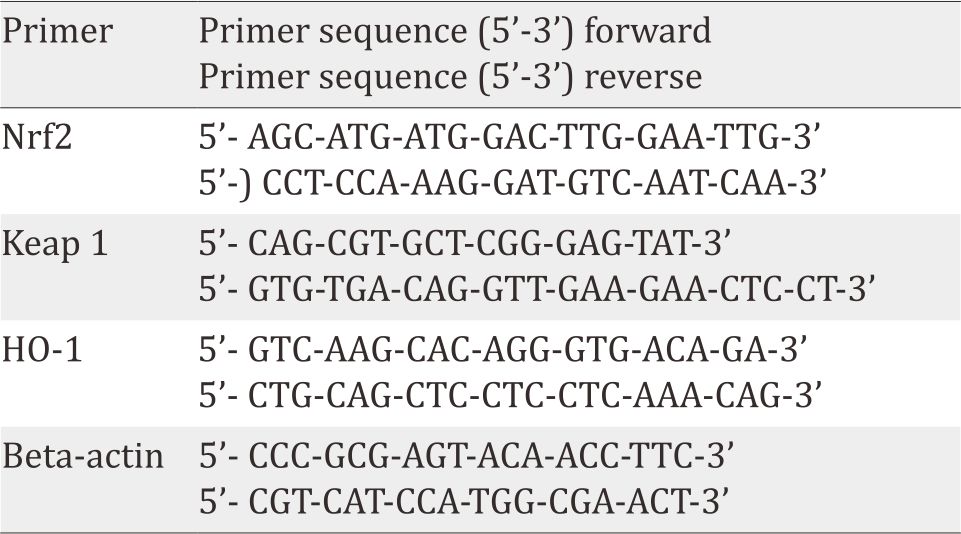
Gene expression analysis was performed by realtime reverse transcription polymerase chain reaction (RT-PCR) using cDNA synthesized from the kidney specimen. Primer sequences were as follows: Relative quantification of real-time RTPCR results were analyzed using Livak method.17
Table 1. Primer sequences
Statistical analysis
All analysis of the experiments were performed in duplicate. Data were expressed as mean ± SD and were analyzed statistically using one-way ANOVA followed by post-hoc (LSD) test. The results were considered statistically significant at p<0.05. If the data were not normal distribution and not homogenous, data were expressed as median, minimal and maximal value and were analyzed statistically by Kruskal-Wallis followed by Mann- Whitney test. The results were considered statistically significant at p<0.05.
RESULTS
Effect of quercetin and captopril on body weight
The survival rate after 5/6 nephrectomy surgery was 57%. After 5/6 nephrectomy, the body weight of all rats tended to decrease. The Nx group had more decreased of body weight compared to the other groups throughout the study period although it was not statistically significant compared to the other groups.
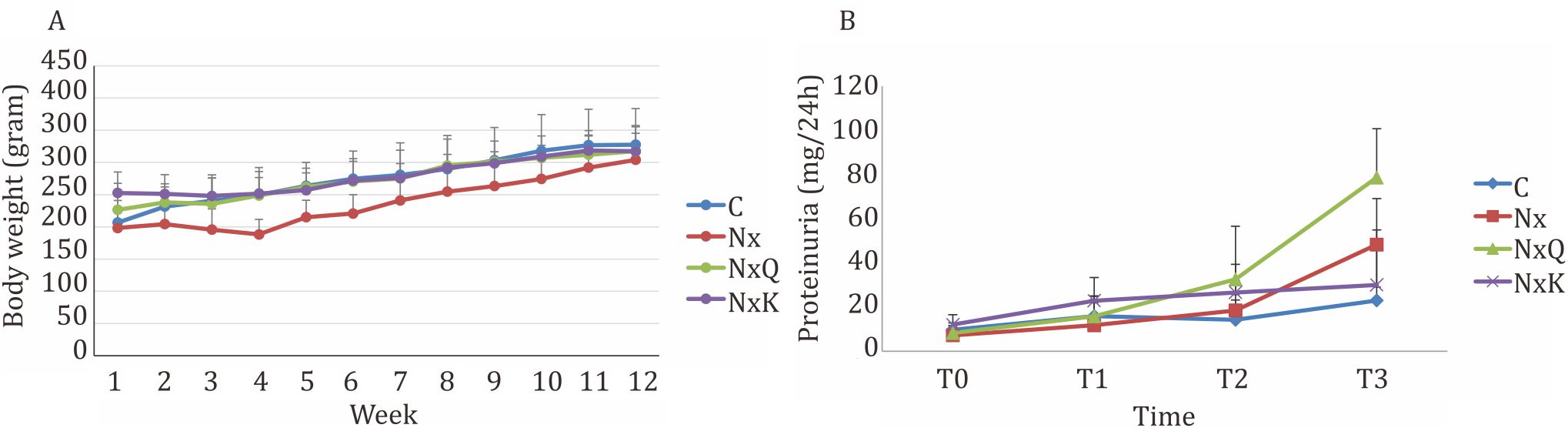
Figure 1. A) Changes in body weight during the study. Neprectomized rats exhibited a reduced of body weight as compared to the other groups. C: normal control; Nx: untreated 5/6 nephrectomized rats; NxQ: nephrectomized rats + quercetin treatment; NxK: nephrectomized rats + captopril treatment; B) Proteinuria was elevated in the 5/6 nephrectomized rats in comparison with the normal control group. At the end of study (week-12), proteinuria significantly increased in Nx, NxQ, and NxK as compared to C (p<0.05). However, proteinuria in the NxQ group still increased as compared to that of the Nx group
Effect of quercetin and captopril on protein in urine, plasma creatinine, and plasma urea
At the end of the study, the Nx group exhibited increased protein in urine, plasma creatinine, and urea as compared to that of the C group. As shown in Figure 2, the Nx group and the NxQ group showed increased protein in urine throughout the study period as compared to the C group while captopril treatment decreased the protein in urine significantly compared to the Nx group and the NxQ group.
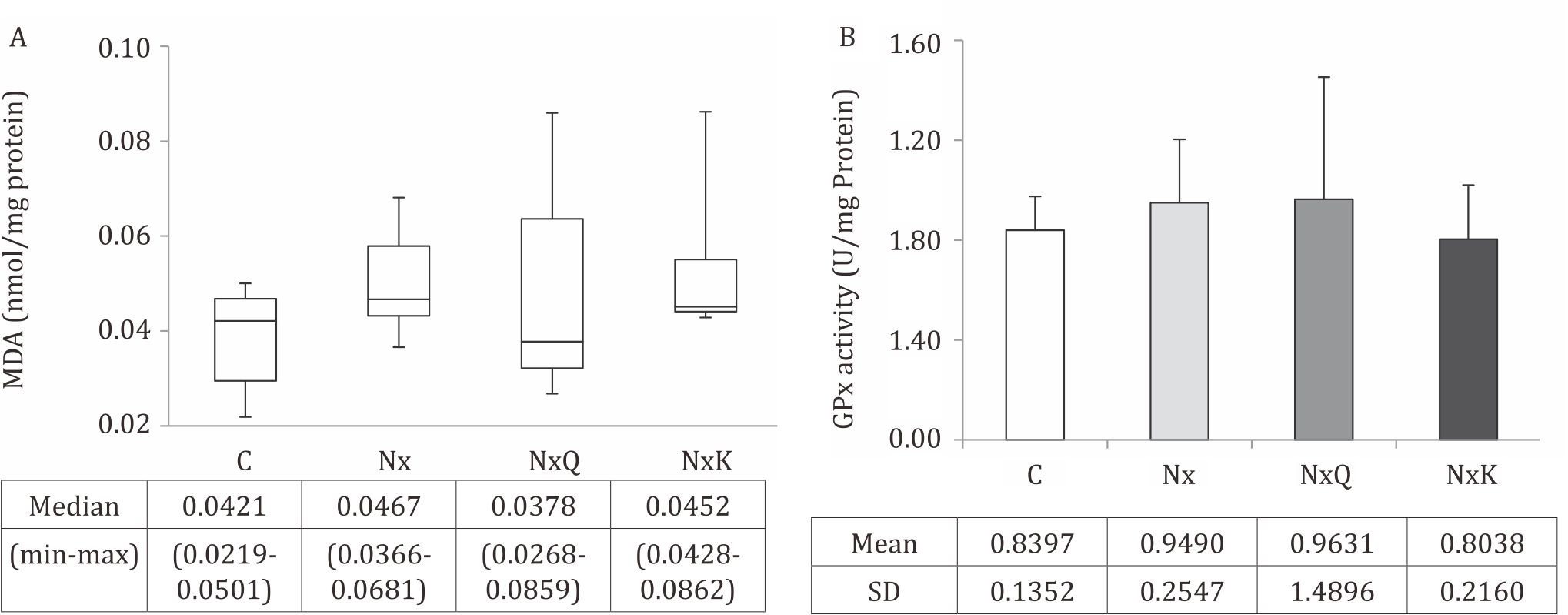
Figure 2. MDA level (A) and the activity of GPx (B) in kidney tissues of the Nx group increased as compared to that of the other groups, though not significantly different (p>0.05). C: normal control; Nx: untreated 5/6 nephrectomized rats; NxQ: nephrectomized rats + quercetin treatment; NxK: nephrectomized rats + captopril treatment; B) in kidney tissues were not different among groups based on ANOVA analysis (p>0.05). C: normal control; Nx: untreated 5/6 nephrectomized rats; NxQ: nephrectomized rats + quercetin treatment; NxK: nephrectomized rats + captopril treatment
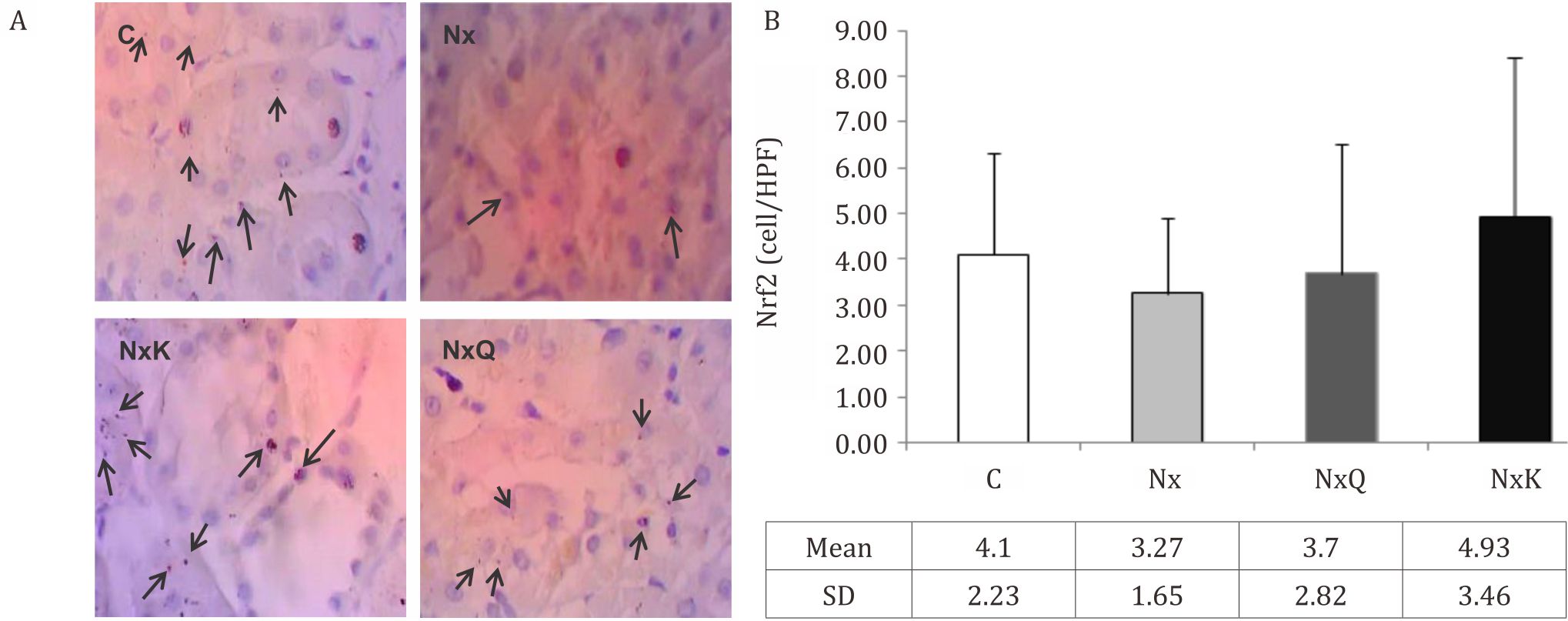
Figure 3. A) Immunohistochemistry staining of Nrf2 in the remnant kidney rats in the Nx group exhibited decreased of Nrf2 positive cells in the nucleus of glomerulus. C: normal control; Nx: untreated 5/6 nephrectomized rats; NxQ: nephrectomized rats + quercetin treatment; NxK: nephrectomized rats + captopril treatment; B) Quantitatively analysis of Nrf2 of immunohistochemistry staining showed that Nrf2 positive cells in the Nx group decreased as compared to the other groups, though not significantly different (p>0.05). C: normal control; Nx: untreated 5/6 nephrectomized rats; NxQ: nephrectomized rats + quercetin treatment; NxK: nephrectomized rats + captopril treatment
Effect of quercetin and captopril on MDA level and GPx activity
Chronic kidney disease has been reported to be related with increased ROS generation and oxidative stress. Kidney lipid peroxidation was determined by MDA analysis. Increased level of MDA and GPx activity in the kidney tissues were found in the Nx group compared with the C group. Treatment with quercetin and captopril decreased the MDA levels. Quercetin treatment also slightly increased the GPx activity although it did not reach a statistically significant compared to that of the Nx group.
Effect of quercetin and captopril on renal expression of Nrf2, Keap1, and HO1
Renal Nrf2 protein expression assessed by immunohistochemistry staining was decreased in the Nx group compared with those in the C group. On the other hand, quercetin and captopril treatment ameliorated these decreases in the Nx group although it did not reach a statistically significant result. In line with protein analysis, renal Nrf2 mRNA expression assessed by RT-PCR was also lower in the Nx group compared with those in the C group. Quercetin and captopril treatment increased the Nrf2 protein and mRNA expression in the kidney tissues. We found that mRNA expression of Keap1 and HO1 were decreased in the Nx group compared with the C group. Treatment with quercetin and captopril increased the mRNA expression of Keap1 and HO1 as well even though it did not show a statistically significant.
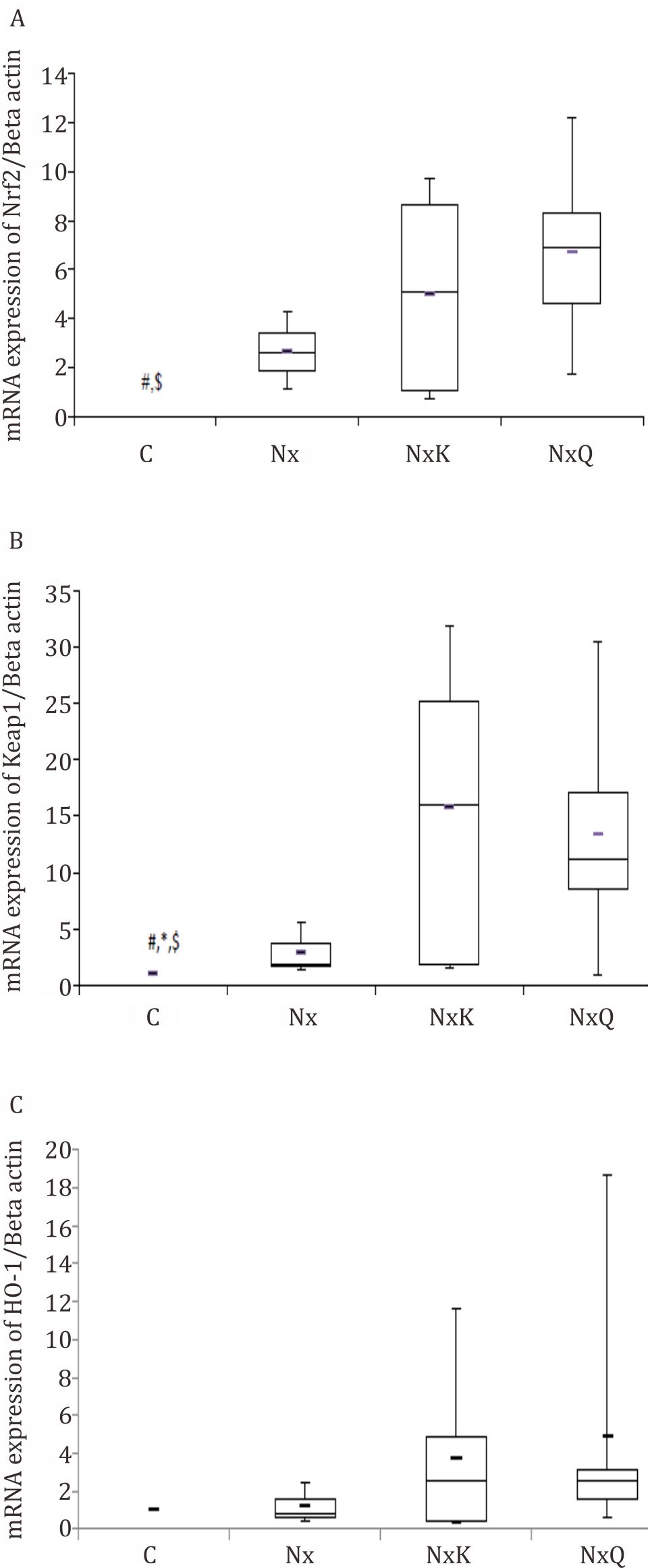
Figure 4. A) mRNA expression of Nrf2 in the Nx group decreased as compared to that of other groups based on Kruskal-Wallis analysis (p=0.039). C= normal control; Nx= untreated 5/6 nephrectomized rats; NxQ= nephrectomized rats + quercetin treatment; NxK= nephrectomized rats + captopril treatment; B) mRNA expression of Keap1 in the Nx group decreased as compared to that of other groups based on Kruskal-Wallis analysis (p=0.011); C) mRNA expression of HO-1 in the Nx group decreased though not significantly different as compared to that of other groups based on Kruskal- Wallis analysis (p=0.413)
Effect of quercetin and captopril on histopathology findings
Histological examination of the kidney of the Nx group had marked histological changes such as sclerosis as shown in the glomerulus (Figure 5A). Figure 5B showed representative Masson’s trichrome staining of glomeruli and tubuli of all groups and the degree of the renal fibrosis. The kidney of the Nx group showed marked interstitial fibrosis. Quercetin and captopril treatment improved slightly the histological changes in the kidney of the Nx group.
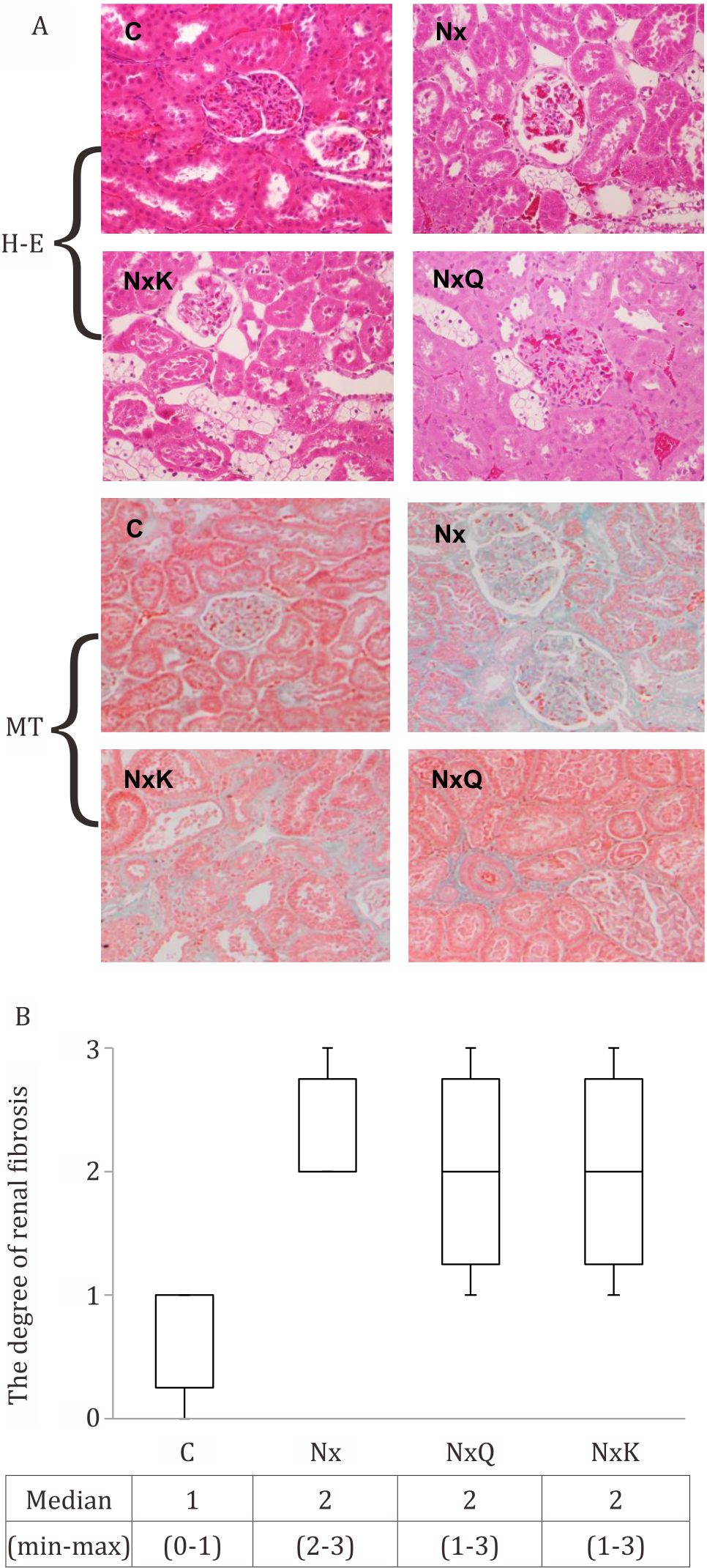
Figure 5. Hematoxyllin-eosin (H-E) staining of the remnant kidney of the Nx group showed glomerulosclerosis as compared to that of the other groups. Masson’s trichrome (MT) staining showed kidney structures in different groups. Fibrosis is indicated by the blue area (magnification x100) in interstitial cortex
DISCUSSION
Chronic kidney disease (CKD) is a progressive disease characterized by a loss of nephron. This condition is associated with a compensatory glomerular hyperfiltration, which increased intraglomerular and triggeredglomerular permeability barrier damage. This leads to protein leakage across glomerular capillaries into Bowman’s space. Protein loss will be reabsorbed by tubules, injured the interstitium tubules cells and finally induced inflammation. This was associated with the upregulation of oxidative stress.2,19
Our study demonstrated that 5/6 nephrectomy, a CKD model, in rats led to proteinuria and increased plasma levels of creatinine and urea. In the present study, we also observed that there was a structural damage of the remnant kidney. This impairment was associated with the increased of oxidative stress as shown by upregulation of MDA levels and slightly reduced GPx activity. Furthermore, it was associated with the decreased expression of Nrf2 and the decreased expression of Keap1 and HO1 which resulted in an increased of fibrotic tissues in the remnant kidney. To assess whether quercetin might have a beneficial role in CKD model, we compared it with captopril. We demonstrated that quercetin could not ameliorate the proteinuria as compared to that of captopril treatment. Quercetin and captopril treatment also could not decrease the plasma creatinine and urea in nephrectomized rats. However, both treatments were able to ameliorate the 5/6 nephrectomy-induced oxidative stress as shown by the decreased of MDA levels, the increased of GPx activity, and the increased of Nrf2, Keap1 and HO1 expression.
It has been shown that captopril could reduce proteinuria more effectively than other antihypertensive. In this study, we also showed that administration of captopril as a comparator to that of quercetin, could reduce proteinuria. Unfortunately, the administration of quercetin was unable to reduce it. In fact, our finding was in agreement with the previous study by Rangan et al20 which showed that quercetin could not reduce protein urine excretion.
Levels of plasma urea and plasma creatinine were used for estimation of renal function. In this eight weeks study after 5/6 nephrectomy, we found that plasma creatinine was increased in the Nx group significantly compared to the C group. Both quercetin and captopril treatments tended to increase plasma creatinine instead of decreased plasma creatinine. This result was similar to the study by Amann et al21 which suggested that 5/6 nephrectomy only slightly increased plasma creatinine. Kim and Vaziri demonstrated that the longer the CKD occured, the worse the increase of serum creatinine.22 Ahmed et al23 demonstrated that the administration of angiotensin converting enzyme inhibitor (ACEI) in the early phase of CKD increased serum creatinine mildly to moderately in patients with deterioration of renal function due to the loss of renal mass which lead to perturbation in the autoregulatory mechanism of the remaining renal vasculature. Subsequently, renal function would either improve or resolve with long-term blood pressure control, reflecting restoration of renal autoregulation towards normal function. ACEI-induced efferent vasodilation also decreased intraglomerular pressure, thereby increased plasma creatinine level24 which might confirm that our 5/6 nephrectomy model was in the early phase of disease. Previous study also showed that ACEI was unable to overcome the uremia.21 Urea is the end product of protein catabolism and digestion. Uremic solutes may have certain characteristics such as large size, large volume of distribution, highly protein bound, able to form crystal deposits, and finally increase production in the uremic state.25 Consistent with the previous study, we demonstrated that both quercetin and captopril treatments were unable to decrease plasma urea.
Chronic conditions of kidney disease will increase reactive oxygen species (ROS) formation in cells. The increase of ROS formation will be balanced by the increase of endogenous antioxidant. Thus, oxidative stress, means imbalance between the prooxidant and antioxidant levels in favor of prooxidant, will not occur. However, longterm increase of ROS formation will impair the antioxidant activity against oxidative stress. In this study, we found that 5/6 nephrectomy increased the malondialdehyde (MDA) levels, products of lipid peroxidation, and the glutathione peroxidase (GPx) activity, an endogenous compound, compared to that of normal group. It suggested that the remnant kidney cells still have the ability to protect against the increased of ROS formation. We also found that quercetin treatment tended to reduce the MDA levels and increase the GPx activity compared to the captopril treatment. In fact, our findings were consistent with the previous study by Shindu et al26 who demonstrated that there were no significant changes of GPx activity in renal insufficiency.
It has been shown that the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element antioxidant pathway has a crucial role in the ability of renal cells to cope with CKD-induced oxidative stress.22 Under unstimulated conditions, Nrf2 is sequestered in the cytoplasma bound to its repressor molecule, Kelch-like ECH-associated protein 1 (Keap1). When exposed to oxidative stress derived from accumulation of ROS, Nrf2 is rapidly dissociated and translocated into the nucleus, inducing gene encoding antioxidant enzymes. In this study, despite oxidative stress which should lead to Nrf2 activation, 5/6 nephrectomy-induced CKD showed reduction of Nrf2 nuclear expression as shown in immunohistochemistry staining. This result confirmed that in 5/6 nephrectomyinduced CKD, some process may halt the translocation of Nrf2 into nucleus. Similar results were found by our group7 and Kim and Vaziri.22 We also have demonstrated that there was an increase of Keap1 and heme-oxygenase-1 (HO- 1) gene expression in the Nx group as compared to the control group. Our results were consistent with other studies conducted by Aminzadeh et al, which was shown that the Nrf2 activation was impaired in rats undergoing CKD due to tubuleinterstitial nephropathy, and it was accompanied by elevation of cytoplasmic Keap1.5 Interestingly, we demonstrated that both quercetin and captopril treatments increased the expression of Nrf2 in the nucleus and increased Keap1 and HO-1 gene expression.
CKD, oxidative stress, and inflammation were related to each other. Renal Fibrosis is the final manifestation of CKD, characterized by tubulointerstitial fibrosis and glomerulosclerosis due to an excessive accumulation of extra-cellular matrix component.27 Our results showed that the degree of fibrosis significantly increased with 5/6 nephrectomy. Both quercetin and captopril treatment ameliorated the degree of fibrosis although the results did not reach statistically significant.
One of the limitations of our study is that the 5/6 nephrectomy surgery was difficult, and the exactly molecular mechanisms which involved in the development of chronic kidney disease was not thoroughly investigated in this study, such as coagulation factors and inflammatory process. Further study with a longer duration of study will elucidate the truly molecular mechanism of quercetin to halt the progression of CKD.
In conclusion, we have demonstrated that quercetin administration reduced oxidative stress and decreased the degree of fibrosis in the remnant kidney of animals with CKD induced by 5/6 nephrectomy, at least in part, through the increased of Nrf2 nuclear expression, Keap1, and HO-1 mRNA expression. However, quercetin could not ameliorate proteinuria and the increased of plasma creatinine and urea. It needs a further study to use queretin as a promising agent to ameliorate the progression of CKD in human.
Conflicts of Interest
Melva Louisa and Vivian Soetikno are editorial board members but were not involved in the review or decision for the article.
Acknowledgment
This research was supported by a grant from the Directorate of Research and Community Services, Indonesia. We thank dr. Tito and dr. Radiana for their assistance in this research work.
REFERENCES
- Quiroz Y, Ferrebuz A, Vaziri ND, Iturbe BR. Effect of chronic antioxidant therapy with superoxide dismutasemimetic drug, tempol, on progression of renal disease in rats with renal mass reduction. Nephron Exp Nephrol. 2009;112:e31–42.
- K/DOQI [Internet]. Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. New York: National Kidney Foundation. [updeted 2008; cited 2016]. Availabel from: https:// www.kidney.org/sites/default/files/docs/ckd_ evaluation_classification_stratification.pdf
- Rivera JR, Ortiz A, Egido J. Antioxidant in kidney disease: the impact of bardoxolone methyl. Int J Nephrol. 2012.
- Kementerian Kesehatan RI. Badan Penelitian dan Pengembangan. Riskesdas 2013. Jakarta. Indonesian.
- Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubuleinterstitial nephropathy. Nephrol Dial Transpl. 2013.
- Glorieux G, Mullen W, Duranton F, Filip S, Gayrard N, Husi H, Schepers E, et al. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol Dial Transplant. 2015; 30: 1842–52.
- Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, et al. Curcumin alleviate oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57:1649–59.
- Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013; 53:401–26.
- Boots AW, Haenen GRMM, Bast A. Health effect of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–37.
- Sriraksa N, Wattanathorn J, Muchimapura S, Tiamkao S, Brown K, Chaisiwamongkol K. Cognitive enhancing effect of quercetin in a rat model of parkinson’s disease induced by 6-hydroxydopamine. Evid Based Complement Med. 2012.
- Lin SY, Wang YY, Chen YC, Chuang YH, Pan PH, Chen CJ. Beneficial effect of quercetin on cholestatic liver injury. J Nut Biochem. 2014.
- Chen SF, Nien S, Wu CH, Liu CL, Chang YC, Lin YS. Reappraisal of the anticancer efficacy of quercetin in oral cancer cells. J Chin Med Assoc. 2013;76:146–52.
- Kleemann R, Verschuren L, Morrison M, Zadelaar S, Erk MJV, Wielinga PY, Kooistra T. Anti-inflammatory, antiproliverative and anti-atherosclerotic effect of quercetin in human In vitro and in vivo models. Atheroscler. 2011;218:44–52.
- Gargouri B, Mansour RB, Abdallah FB, Elfekih A, Lassoued S, Khaled H. Protective effect of quercetin against oxidative stress caused by dimethoate in human peripheral blood lymphocytes. Lipids Health Dis. 2011.
- Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and keap 1 in ARE-medaited NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–703.
- Wang C, Pan Y, Zhang QY, Wang FM, Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7(6):e38285.
- Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013 Nov;50(6):1007-15.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2-ΔΔC T method. Methods. 2001;25:402–8.
- Fedorova LV, Tamirisa A, Kennedy DJ, Haller ST, Budnyy G, Shapiro JI, et al. Mitochondrial impairment in the five-sixth nephrectomy model of chronic renal failure: proteomic approach. BMC Nephol. 2013;14:209.
- Rangan GK, Wang Y, Harris DCH. Dietary quercetin augment activator protein-1 and doesn’t reduce NF- κB in the renal cortex of rats with established chronic glomerular disease. Nephron. 2002;90:313–9.
- Amann K, Gassmann P, Buzello M, Orth SR, Tornig J, Gross ML, et al. Effect of ACE inhibition and bradykinin antagonism on cardiovascular changes in uremic rats. Kidney Int. 2000;58:153–61.
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2- Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–71.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010 Dec;25(12):3977-82.
- Palmer BF. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers: what to do if serum creatinine and or serum potassium concentration rises. Nephrol Dial Transplant. 2003;18:1973–5.
- Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–25.
- Sindhu RK, Ehdaie A, Farman F, Dhaliwal KK, Nguyen T, Zhan CD, et al. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim Biophys Acta. 2005:86–92.
- Cho MH. Renal fibrosis. Korean J Pediatr. 2010;53(7):735–40.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id