
Section Abstract Introduction Methods Results Discussion Conflict Of Interest Acknowledgment References
Clinical Research
The effect of Bifidobacterium animalis lactis HNO19 supplementation among pregnant and lactating women on interleukin-8 level in breast milk and infant’s gut mucosal integrity
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i3.1481 Med J Indones. 2017;26:204–11
Received: June 22, 2016
Accepted: July 06, 2017
Author affiliation:
1 Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Child Health, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Department of Obstetric Gynecology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Pediatric, Budi Kemuliaan Hospital, Jakarta, Indonesia
5 Department of Pathology Clinic, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
6 Department of Nutrition, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
7 Department of Child Health, Faculty of Medicine, Universitas Padjadjaran, Sumedang, Indonesia
Corresponding author:
Naomi E.F. Dewanto
E-mail: naomiesthernita@gmail.com
Background
Newborn’s gut mucosal is not fully developed, therefore infants are prone to diarrhea. Probiotic supplementation is known to induce the gut mucosal maturity. This study aimed to identify whether probiotics supplementation among pregnant women since the third trimester would increase the infant’s gut mucosal integrity.
Methods
A double-blind, randomized clinical trial was conducted to understand the potential effect of probiotic supplementation on the level of probiotics and IL-8 in breastmilk, urine IFABP, faecal α-1-antytripsin (AAT) and calprotectin in infant’s at birth (V0) and three-months old (V3). A single strain of Bifidobacterium lactis animalis HNO19 (known as DR10) was used since it was not the resident bacteria. The study was held at Budi Kemuliaan Hospital and its satellite clinics from December 2014 to December 2015.
Results
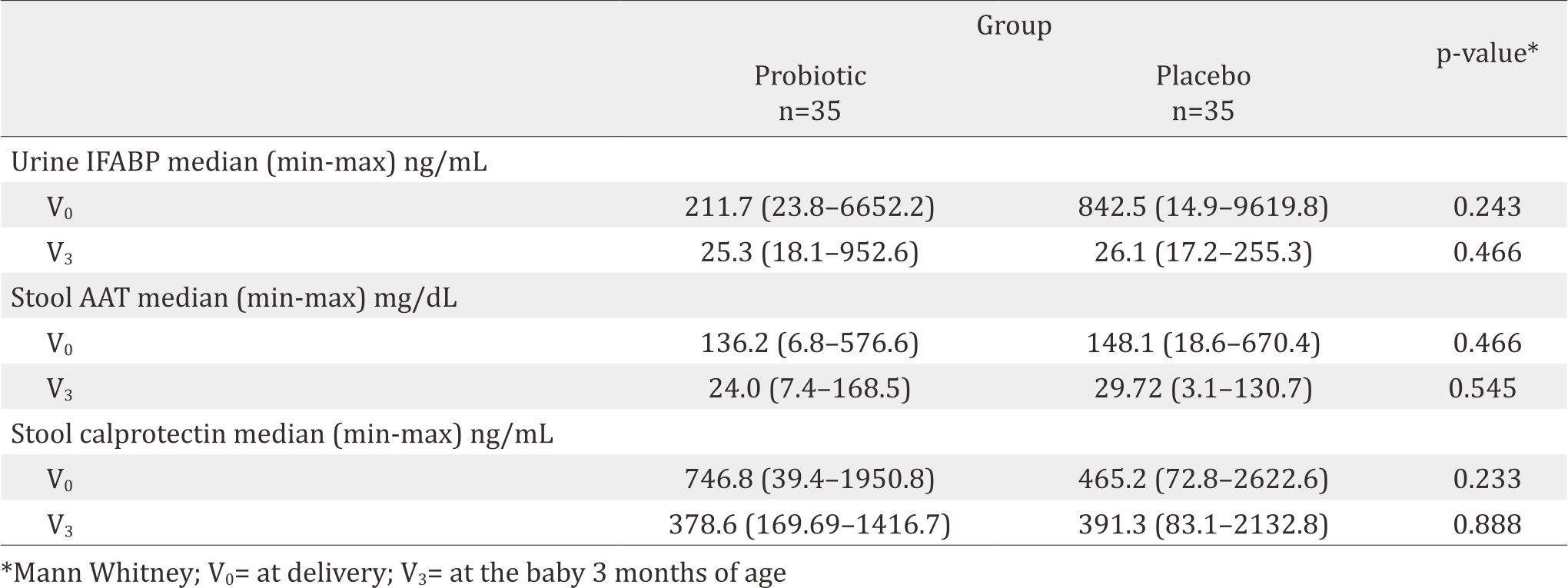
About 14% (5/35) and 20% (7/35) of the subjects had DR10 in the breastmilk’s colostrum and at the age of 3-months. The median values of IL-8 in the probiotic group vs the placebo group at V0 and V3 were 2810,1 pg/mL vs 1516.4 pg/mL (p=0.327) and 173.2 pg/mL vs 132.7 pg/mL (p=0.211) respectively. IFABP level 211.7 ng/mL vs 842.5 ng/mL (p=0.243) and 25.3 ng/mL vs 25.1 ng/mL (p=0.466); AAT 136.2 mg/dL vs 148.1 mg/dL (p=0.466) and 24 mg/mL vs 29.72 mg/mL (p=0.545); Calprotectin 746.8 ng/mL vs 4645.2 ng/mL (p=0.233) and 378.6 ng/mL vs 391.3 ng/mL (p=0.888).
Conclusion
Probiotic DR10 given to pregnant women since the 3rd trimester can be found in colostrum and 3-months breastmilk. However, it did not affect the level of other probiotics or IL-8 and the gut mucosal integrity.
Keywords
Bifidobacterium lactis animalis HNO19, breastmilk, gut mucosal integrity
The infant’s intestinal mucosal growth is not mature, yet this is called “leaky gut”. The intestinal mucosal develops until the infants reach two years of age. Therefore, the highest incidence of diarrhea is found among children under two years old. The mucosal epithelial barrier and immunoregulatory network are poorly developed in newborns. The infant’s abrupt introduction to life outside uterus and the exposure to antigens force the gastrointestinal (GI) tract to adapt quickly and commence its crucial duties.1
However, the neonate’s adaptive immune system is still immature, leaving the newborn in a state of vulnerability and at increased risk for serious infection.2 Human milk, the natural infant feeding, is a complex species-specific biological fluid adapted to perfectly satisfy the nutritional and immunological needs to neonate. The compounds presented in colostrum or mature milk may have anti-infective effect.3
Recently, extensive research has been conducted to understand the beneficial role of human milk oligosaccharides (HMO), introducing the new concept of the ‘milk microbiome’. The mechanism of whether bacteria contamination or active migration helps bacteria to reach mammary gland is debatable4 and further study is necessary. Numerous studies had identified probiotics in breastmilk.3,5 Probiotic consumptions may help other bacteria to grow, for example supplementation of Bifidobacteria affects Lactobacillus growth.6 Administration of Enterococcus faecium CRL 183 increases Bifidobacteria and short chain fatty acid (SCFA) levels whereas Lactobacillus acidophilus CRL 104 increases Bifidobacterium and Lactobacillus, as well as acetate in an in vitro GI model.7 Interleukin 8 (IL-8) presents in significant amount in human milk. Studies showed that a consistent increase in cell migration, proliferation, and differentiation occured when human fetal and adult intestinal cells were treated with rhIL-8 in vitro.8 Other in vitro study found the relationship of E. coli LTH 634 and Lactobacillus sakei with the production of IL-8.9
This study aims to provide evidence whether probiotics are present in breastmilk and affect the growth of other bacterias, and also increase the IL-8 production and mucosal integrity of infants, compared to the placebo group. A single strain of Bifidobacterium lactis animalis HNO19, a non-resident bacterium, was used in this study.
METHODS
Study design
A double-blind, randomize controlled trial with two parallel groups was conducted to understand the effect of probiotic supplementation in pregnant women, assessing the IL-8 production in breast milk, urinary intestinal fatty acid binding protein (IFABP), α-1-antytripsin (AAT) and calprotectin in feses at birth and three-month old infant. This study was conducted from December 2014 to December 2015 at Budi Kemuliaan hospital and its satellite clinics. A total of 110 pregnant women in their third trimester, who came to the obstetrics and gynecology outpatient department in the selected hospitals were enrolled. The inclusion criteria were pregnant women at third trimester, normal pregnancy, plan to deliver spontaneously, no consumption of antibiotics, and plan to exclusively breastfeed the baby (at least until the baby is three months old). The newborns of the study were also included in the study. Exclusion criteria were pregnant women with pre-eclampsia, bleeding, infection, premature rupture of the membrane or other chronic disease. This study was approved by the Health Research Ethics Committee Faculty of Medicine University of Indonesia number 525/UNS.F1/ETIK/2014.
Sample selection
Pregnant women who fulfilled the inclusion criteria at Budi Kemuliaan Hospital and its satellite clinics would be recruited. They were asked to participate in the study and signed the informed consent. The subjects were divided into two groups by block permutted randomization with the size of ten. The subjects were restricted to consume food/drink with probiotic ingredients, such as yoghurt and yakult, during the study. Their diet was controlled by nutrition experts.
Intervention stage
The treatment group was given probiotic capsul of Bifidobacterium lactis animalis HNO 19 with the dose of 109 unit every day until their newborns were three-months old. While for the same period, the control group was given placebo daily. In case of bleeding during birth delivery or cesarean surgery or antibiotics treatments, the subjects would be excluded from the study (dropped out).
Initial breastmilk in the first days after birth (colostrum) was obtained and examined using real time (RT) polymerase chain reaction deoxyribose-nucleic acid (PCR DNA) to identify the total number of microbiota, genus Bifidobacteria, genus Lactobacillus, and strain Bifidobacterium lactis animalis HNO19 (DR10). A manual sampling was conducted; the nipples and mammary areole were cleaned with antiseptic. The researcher washed their hands and wore sterile gloves before doing the examination. Skin swab around the breast was also performed. Infants were examined for intestinal fatty acid binding protein (IFABP) in their urine and for calprotectin, alpha 1 antytripsin, in their stool. The gestational age, anthropometric status (birth weight, birth length, head circumference), mode of delivery, and Apgar score were recorded from the newborns. Infants with respiratory distress, asphyxia, seizures, congenital abnormalities, require NICU or were not able to drink orally are considered as drop-out (DO). During breastfeeding, subjects were monitored and counseled by a breastfeeding counselor in order to achieve exclusive breastfeeding. Same procedures were performed when the infants reached threemonths old.
Specimen collection and examination of breastmilk
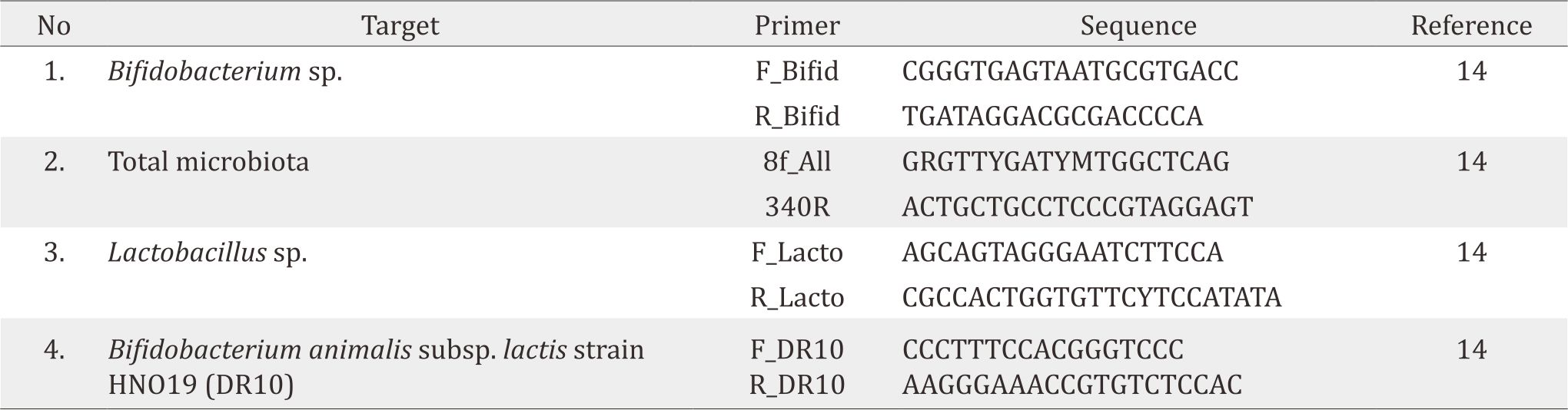
Colostrum were obtained after birth delivery (day 1–5). Trained persons collected the samples with aseptic and antiseptic action. After pumping, at least 0.1 mL of breastmilk was poured into a sterile tube containing 300 mg of zirconium beads (diameter 0.1 mm). Afterwards, the samples were centrifuged several times in the laboratoray, washed with ethanol, and centrifuged again before DNA separated. Real-time PCR was performed using the applied biosystems (ABI) 7500 Fast Sequence Detection System using software version 2.0 (Applied - Biosystems). Primers were designed based on 16S ribosa ribonucleic acid (rRNA) specific for Bifidobacterium sp., total bacteria, Lactobacillus sp. and Bifidobacterium lactis animalis HNO19 (Table 1). Each reaction run with duplication of the final volume 15 μl with a final concentration of 0.3 to 0.9 μM each primer, and 10 mL of appropriate dilution with the DNA sample. IL-8 in breastmilk was also examined using enzyme linked imunosorbent assay (ELISA) method.10 Before collecting breastmilk, we did skin swab around the breast and examined for DR 10.
Table 1. Primer quantitative PCR for microbiota in breastmilk.14
Examination of urine IFABP, stool AAT and calprotectine
In this study, I-FABP urine was measured by Elisa using human I- FABP ELISA kits HK406.11 One mL urine was collected with aseptic procedure and removed to the poliprophylen tube, which then kept in -80°C storage. Before analysis, the specimens were removed to -20°C for one night, then they were removed again to room temperature (18–25°C), and mixed well. Centrifugation was conducted to remove the debris, then everything was done as in the kit procedure.
AAT and calprotectin was measured in stool.12,13 Make sure reagen and specimen were mixed adequately. Put 100 mg stool to a plastic vial then add 5 mL Exbuff. Centrifugation and dilution for AAT and calprotectin were performed differently according to the kit procedure.
Data Analysis
All data were analysed using statistical product and service solutions (SPSS) version 20 and presented in text, tables, or graphs. We used 95% of confidence level 80% power. Data with normal distribution presented mean and standard deviations values while data with abnormal distribution used median values. Mann Whitney and independent t-test were applied in this study.
RESULTS
About 110 subjects were recruited and enrolled to this study, but 40 of them were dropped out, leaving only 70 subjects (Figure 1). Primer quantitative PCR for microbiota in breastmilk showed in Table 1. The majority were housewives, aged 20–29 years, giving birth once, graduated from high school, and received income of 2–9 million IDR (Table 2). About 14% (5/35) subjects in probiotic group had DR10 positive in their breast milk during birth delivery (V0) and 20% (7/35) at three-months postpartum (V3). However, none were positive in the placebo group, both during birth delivery and at three-month postpartum. Skin swab indicated negative result in all groups (Table 3). Composition of microbiota and IL-8 in breastmilk showed in Table 4. Result of urine IFABP, stool AAT and calprotectin showed in Table 5.
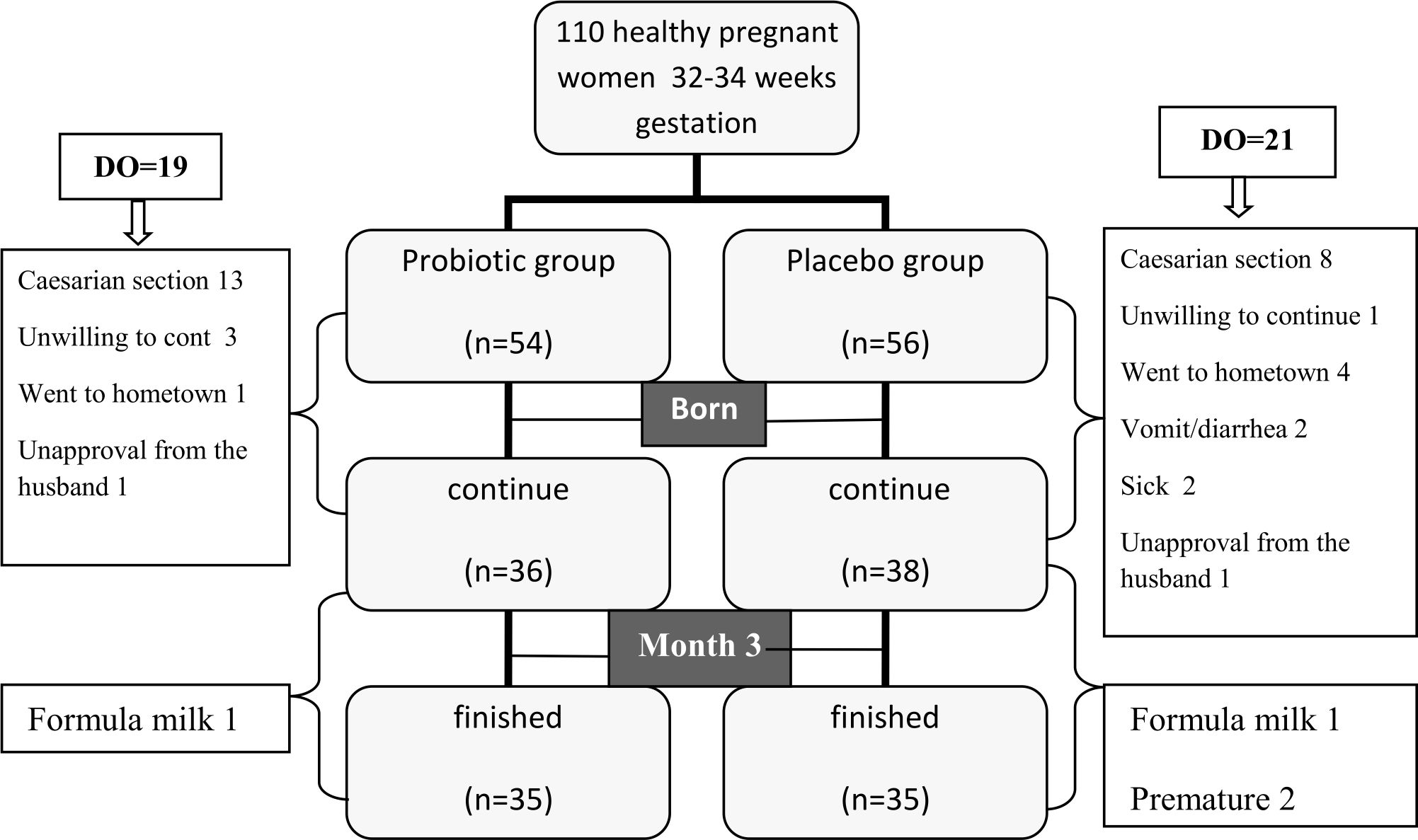
Figure 1. Subject recruitment. DO= drop out
Table 2. Subject charasteristic
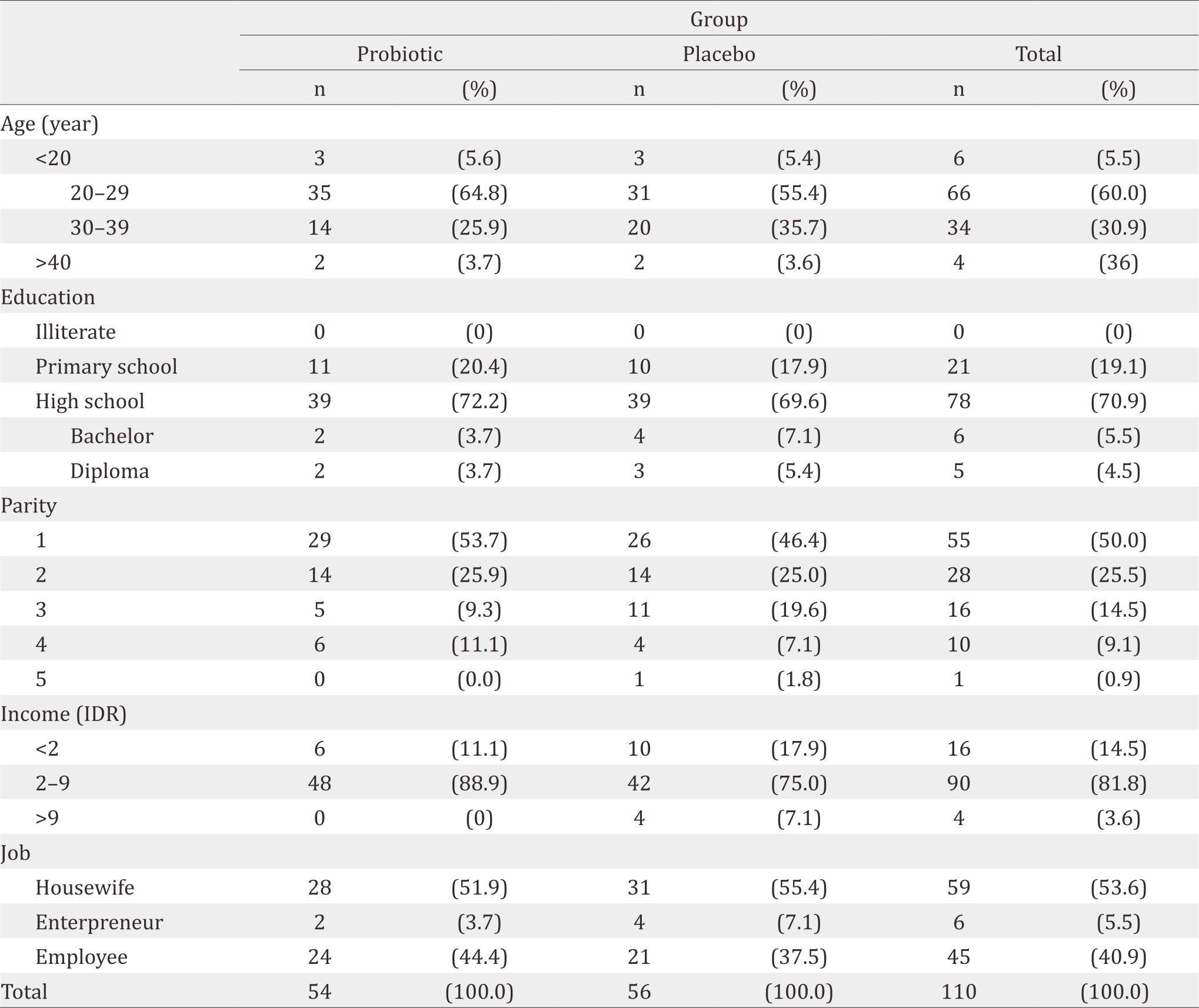
Table 3. Bifidobacterium lactis HNO19 in breast milk and skin swab
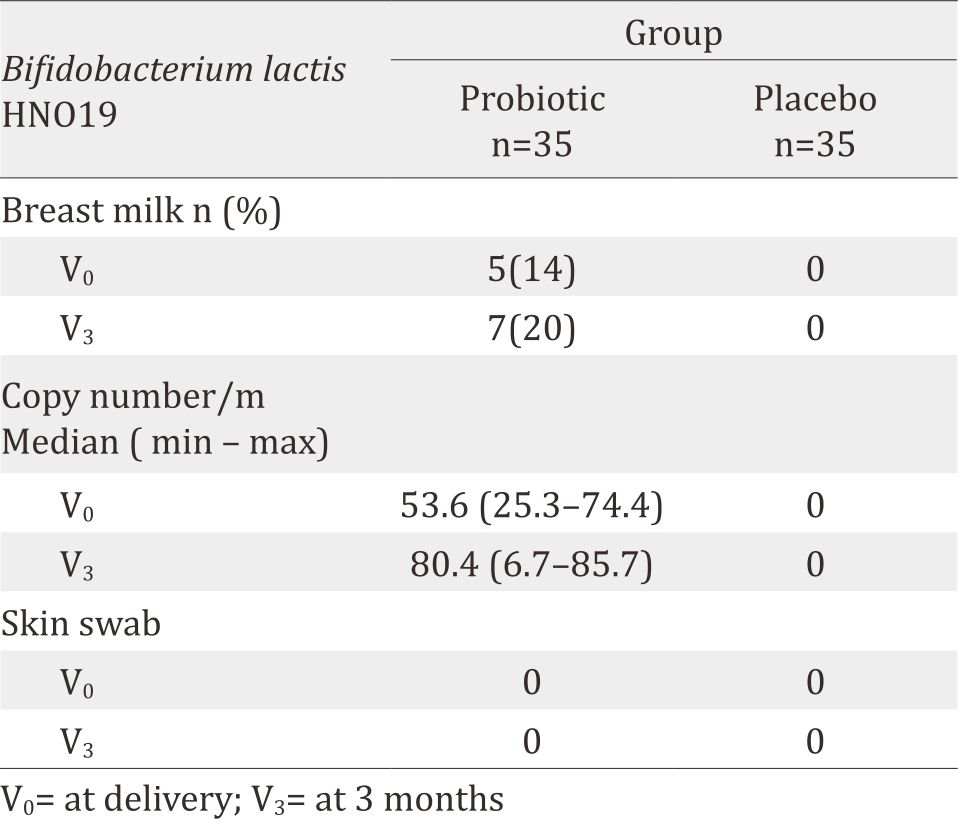
Table 4. Composition of breast milk microbiota and level of IL-8 in breast milk
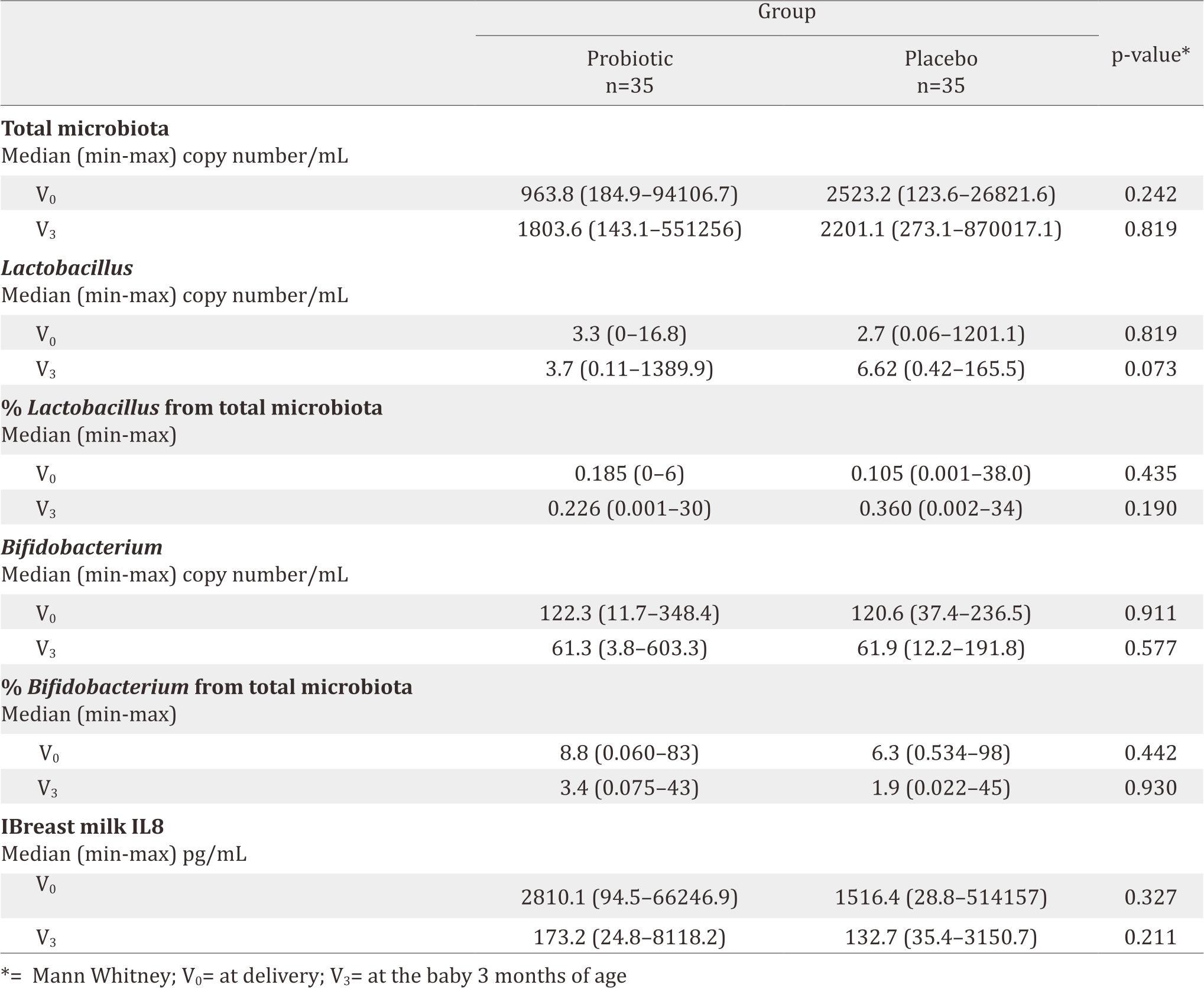
Table 5. Urine IFABP, stool AAT and calprotectin
DISCUSSION
This is the first study explaining the correlation between probiotic diet and breastmilk microbiota among women after birth delivery in Indonesia. Many factors influence the viabillity and survival of probiotic in breastmilk, such as genetic, culture, environment, diet, mode of delivery, and antibiotic treatment during pregnancy and lactation.15 Breastmilk of women who received antibiotics during pregnancy or lactation had lower content of Lactobacilli or Bifidobacteria compared to women who received no antibiotics.16 In this study, both the intervention and the placebo group had Lactobacillus and Bifidobacterium in their breastmilk. Subjects who received antibiotics during pregnancy had been excluded from this study. One subject with premature rupture of membrane was excluded due to caesarean section. There were few subjects who received antibiotics during delivery or maximum five days postpartum via oral or three days via parenteral. However, their test were still positive. These might happen because the microbiota depression period ranges usually between 3–8 weeks while the time period between V0 and V3 examination was three months. Our findings also support that Bifidobacterium animalis lactis HNO19 (DR 10) is transient microbiota that can be transferred from mother to fetus through mammary lymph nodes.17,18 After consumption, ingested bacteria enter a hostile environment where subsequent passage through stomach and duodenum exposes them to highly stressful physiochemical and biological conditions such as gastric acid and bile salt.15 DR 10 that reach gastric lumen are taken up by dendritic cell (DC) to the mesenteric lymph nodes, respiratory tract, genitourinary tract, salivary and lacrimal glands, and eventually mammary gland. DCs and macrofags are able to discriminate between pathogenic and non-pathogenic compounds through the expression of various pattern-recognition receptors (PRRs).17,18 Bacterial signaling on the mucosal surfaces is dependent on the network between bacteria, epithelial cells, and the immune system. Therefore, not all subjects in the probiotic group had positive DR 10 in their breastmilk.
The composition of breastmilk microbiota is complex. From the hundreds of operational taxonomic units (OTUs) detected in the milk of every woman, only nine were present. Surprisingly, these nine “core” OTUs represented about half of the microbial community observed. Infant’s immune system was greatly influenced by their maternal immunity which transferred via placenta and breast milk. IL-8 in breastmilk indicated the leukocyte movement from mothers to infants. The levels of cytokine were high in colostrum and transient milk; then it would reduce in the first 21 days and 60 days of breastmilk production.19 This study confirmed that IL-8 level in colostrum was found higher in the probiotic group than in the placebo group after birth (V0), but their level of IL-8 was equal in the probiotic as well as the placebo group at three-month postpartum. Probiotics did not affect the chemokine content from colostrum to mature milk.
Intestinal fatty acid binding protein (IFABP) is an indicator of enterocyte damage.20 In this study, the median IFABP level at delivery (V0) was lower in the probiotic group than in the placebo group (p=0.243). This means less enterocyte damage happened in the probiotic group. The low level on the probiotic group at V0 seemed to correlate with the high level of IL-8, which improved gut maturity, then decreased enterocyte damage although it was not significant. During breastfeeding, the guts had microbiota to protect enterocyte and therefore, no differences were found in terms of IFABP level in both the probiotic and the placenta group 3-month after birth. The supplementation of DR10 had no effect to make any difference on IFABP level.
AAT is a serum protein that is resistant to enzymatic proteolysis in the gastrointestinal tract. This protein does not exist in the diet. Since this protein is excreted, testing the content of AAT in faeces could reflect protein entering the intestine from the intravascular space. Faecal AAT has been considered as a reliable and inexpensive method for the estimation of enteric protein loss.21 The AAT level in both group at birth was similar, meaning that DR10 supplementation showed no effect in reducing enteropathy. While in the age of 3 months, the AAT level was decreased in both groups since the breastmilk already contained cytokine, secretory Ig A, and fatty acids.
Calprotectin, calcium and zinc binding protein, presents in monocytes, macrophages, and epithelial cells. Its function is to regulate inflammatory processes.22 High faecal calprotectin levels correlate with an increased turnover of leukocytes in the intestinal mucosa and granulocyte migration to intestinal lumen. Faecal calprotectin levels have been reported to be much higher during the first few weeks of life, both in healthy full-term and pre-term infants. The gut mucosa in newborn infants tends to have higher risk of inflammation.23 In gut inflammation, calprotectin can be detected in stool and plasma. Therefore, stool calprotectin could be used as a good marker for necrotizing enterocolitis (NEC). The calprotectin level in this study were both decreasing and found to have similar level at V3. This might happened because breastmilk naturally had the ability to reduce inflammation in gut mucose. Longer follow-up after delivery is necessary to explore and analyse the effect of probiotics to the gut mucosal integrity and its role to improve gut mucosal integrity by comparing subjects with and without probiotics.
A high number of dropped-out subjects was found in this study because of unexpected cesarean section and low compliance. The socio-economic condition of the subjects may influence their compliance. Dropped-out due to formula milk usage was relatively low. In the first three months after birth, the rate of exclusive breastfeeding was still high. The same condition was found by Collado et al24 babies who got breast milk at delivery, 66.1% of them continued to give exclusive breastfeeding until 6 months postpartum. However, the average exclusive breastfeeding duration is three months. The data from Basic National Research (Riskesdas) in 2012, 42% of 0–6 months exclusive breastfeeding.25
In conclusion, probiotic Bifidobacterium animalis lactis HNO19 (DR10) given to pregnant women since the 3rd trimester can be found in colostrum and breastmilk (three-months postpartum). However, it did not affect the level of other probiotics or IL-8 and the gut mucosal integrity
Conflicts of Interest
The funding and source of probiotic strain were supported by Friesland Campina Innovation and Fontera.
Acknowledgment
The author is thankful to all people who were involved in this study especially dr. Yenny Tirtaningrum, dr. Yolanda Savitri, dr. Sri Nindita and all the midwives at Budi Kemuliaan Hospitals. I would like to express my thanks to Friesland Campina Innovation for providing financial assistance for my study. Thanks are also due to Fontera for suppliying the probiotic strain Bifidobacterium animals lactis HNO19 for the study.
REFERENCES
- Burrin DG. Physiology of gastrointestinal tract in fetus and neonate. Dalam: Polin RA, Fox WW, Abman SH, eds. Fetal and neonatal physiology. 4th ed. USA: Elsevier Saunders; 2011:1181.
- Jakaitis BM, Dening PW. Human breast milk and gastrointestinal innate immune system. Clin Perinatol. 2014;41(2):423–35.
- Villoslada FL, Olivares M, Sierra S, Rodriguez JM, Boza J, Xaus J. Benneficial effects of probiotic bacteria isolated from breast milk. Brit J Nutr. 2007;98:S96–S100.
- Jeurink PV, Bergenhenegowen JV, Jimenez E, Knippels LM, Fernaindez L, Garssen J et al. Human milk: a source of more life than we imagine. Benef Microbes. 2013;4(1):17–30.
- Martin R, Jiminez E, Heilig H, Fernandez L, Martin ML, Zoetendal EG et al. Isolation of Bifidobacteria from breast milk and assessment of the Bifidobacterial population by PCRDeanturing gradient gel electrophoresis and quantitative real-time PCR. Appl Enviro Microbiol. 2009;75:965–9.
- Ahmed M, Prasad J, Gill H, Stevenson L, Gopal P. Impact of consumption of different levels of Bifidobacterium lactis HNO 19 on the intstinal microflora of elderly human subjects. J Nutr Health Aging. 2007;11(1):26–31.
- Derrien M, Vlieg JET VH. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23(6):354–66.
- Maheshwari A, Lu W, Lacson A, Barleycorn AA, Nolan S, Christensen RD, et al. Effects of interleukin- 8 on the developing human intestine. Cytokine. 2002;20(6):256–68.
- Lammers KM, Helwig U, Swennen E, Rizzello F, Venturi A, Caramelli E, et al. Effect of probiotic strains on interleukin 8 production by HT29/19A cells. Am J Gastroesterol, 2002; 97(5):1182–6.
- HK 310 Human IL-8 Elisa Kit. Product Information and Manual, Vol. 02-10. p. 1–14.
- Hycult Biotech. HK 406 human I-FABP ELISA kit. Product information and manual. Edisi 08-13, p.1–14.
- Manual of α-1 antitrypsin ELISA. EIA-5299. Version: 4.0. Feb 2015.
- Human Kalprotektin in Stool ELISA. Protokol. Pediatric Research Unit. Departemen Ilmu Kesehatan Anak. FKUI 2009. Indonesian.
- Oswari H, Prayitno L, Dwipoerwantoro PG, Firmansyah A, Makrides M, Lawley B, et al. Comparison of stool mikrobiota compositions, stool alpha1-antitrypsin and calprotectin concentrations, and diarrhoeal morbidity of Indonesian infants fed breast milk or probiotic/prebiotic- supplemented formula. J Paediatr Child Health. 2013;49(12):1032–9.
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78(1):80–8.
- Soto A, Martín V, Jiménez E, Mader I, Rodríguez JM, Fernández L. Lactobacilli and Bifidobacteria in human breast milk: influence of antibiotherapy and other host and clinical factors. J Pediatr Gastroenterol Nutr. 2014;59(1):78–88.
- Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9(10):565–76.
- Collado MC, Rautava S, Salminen S, Isolauri E. Gut mikrobiota: a source of novel tools to reduce the risk of human disease? Pediatr Res. 2015;72(1–2):182–8.
- Ustundag B, Yilmaz E, Dogan Y, Akarsu S, Canatan H, Halifeoglu I, et al. Levels of cytokines (IL-1beta, IL-2, IL- 6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm. 2005(6):331–6.
- Gregory KE, Winston AB, Yamamoto HS, Dawood HY, Fashemi T, Fichorova NR, et al. Urinary IFABP predicts necrotizing enterocolitis within seven days prior to clinical onset. J Pediatr. 2014;164:1486–8.
- Tangsilsat D, Atamasirikul K, Treepongkaruna S, Nathsevee S, Sumritsopak R, Kunakom M. Faecal alpha –antitrypsin in healthy and intestinal disorder Thai children. J Med Assoc Thai. 2007;90:1317–22.
- Rouge C, Butel MJ, Piloquet H, et al. Faecal calprotectin excretion inpreterm infants during the neonatal period. PLoS One. 2010;5(6):1–6.
- Kapel N, Campeotto F, Kalach N, Baidassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr. 2010;51:542–7.
- Collad MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res. 2012;72:77–85.
- Badan Penelitian dan Pengembangan Kesehatan Kementerian Kesehatan RI [Internet]. Riset kesehatan dasar 2013. [update 2013; cited January 2015] Available from: http://www.riskesdas.litbang.depkes.go.id/2013. Indonesian.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id