
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Clinical and ultrasound joint outcomes in severe hemophilia A children receiving episodic treatment in Indonesian National Hemophilia Treatment Center
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i1.1494 Med J Indones. 2017;26:47–53
Received: July 01, 2016
Accepted: December 29, 2016
Author affiliation:
1 Department of Child Health, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Medical Rehabilitation, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Department of Pathology Clinic, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Internal Medicine, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
5 Department of Nutrition, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
6 Department of Child Health, Hasan Sadikin Hospital, Faculty of Medicine, Universitas Padjajaran, Bandung, Indonesia
7 Department of Radiology, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Novie A. Chozie
E-mail: novie.amelia@ui.ac.id
Background
Recurrent joint bleeds leading to arthropathy is the main problem in severe hemophilia children. This study aimed to investigate joint status in severe hemophilia A children receiving episodic treatment in Cipto Mangunkusumo Hospital, Jakarta.
Methods
A cross-sectional study was conducted in Cipto Mangunkusumo Hospital as Indonesian National Hemophilia Treatment Center on children (4–18 years) with severe hemophilia A, who previously received episodic treatment, with no history of inhibitor factor VIII. Hemophilia Joint Health Score was evaluated according to HJHS version 2.1 2011. Joint ultrasonography was done for six index joints (bilateral elbows, knees and ankles) using Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) methods. Data of age of first joint bleed, number of target joints and inhibitor factor VIII were obtained from the Pediatric Hemophilia Registry and medical records.
Results
There were 59 subjects aged 4 to 18 years. Twentynine out of 59 (49.2%) subjects experienced first joint bleed before of 2 years of age. The most common of joint bleeds was a right ankle. Mean total HJHS was 8.71±8.73. Subjects aged 4–10 years showed lower HJHS (4.6±3.7) as compared to subjects aged >10–18 years (12.3±10.3), p<0.001; 95% CI=4.9–13. Mean HEAD-US scores in subjects aged 4–10 years (18.7±5.6) was lower than in subjects aged >10–18 years (28±7.9), p<0.001, 95% CI= -12.9–-5.6.
Conclusion
HJHS and HEAD-US scores of severe hemophilia A children receiving episodic treatment aged 4–10 years are lower compared to subjects aged >10–18 years, indicating more severe joint destruction in older children and progressivity of joint damage over time. It is important to start prophylactic treatment to prevent progressivity of joint damage.
Keywords
arthropathy, HEAD-US, hemophilia, HJHS, inhibitor factor VIII
Hemophilia A (factor VIII deficiency) is the most common inherited bleeding disorder in the world. The typical clinical manifestation of severe hemophilia is spontaneous joint bleeding.1 Repeated joint bleeding will lead to irreversible progressive damage of the joint (hemophilic arthropathy).2
Hemophilia Treatment Center in Jakarta, Indonesia. Hemophilia treatment practice in Indonesia as a developing country is currently improving from the wide use of cryoprecipitate to factor concentrates as episodic treatment but not prophylaxis. Prophylaxis has not become the standard of treatment due to very expensive cost.3 With episodic treatment, risk of developing hemophilic arthropathy in adulthood is very high and most patients will suffer from chronic pain,2,4 physical disability and psychosocial problems in adulthood.5 To monitor progressivity of hemophilic arthropathy, routine monitoring with clinical and radiological measurement is very important in hemophilia management.2 International Prophylaxis Study Group (IPSG) has developed Hemophilia Joint Health Score (HJHS), that has good sensitivity and can be applied according to child developmental stage.6 HJHS evaluates structure and function of bilateral knee, elbow and ankle (total six joints).6–8 Currently the best joint imaging to detect early joint damage is magnetic resonance imaging (MRI), but the cost is expensive, not available in every center and needs sedation for children.9 Ultrasound has been used to assess the joint swelling and differentiate acute bleeding or chronic synovitis without acute bleeding.10 Martinoli et al11 developed ultrasound procedure and scoring method named Haemophilia Early Arthropathy Detection with Ultrasound (HEADUS) to evaluate knee, elbow, and ankle joint in order to improve sensivity in detecting early sign of arthropathy, using systematical, easy, and fast technique. Joint ultrasound is a potential tool for monitoring hemophilic arthropathy in a developing country as Indonesia, because it is less expensive, widely available, and has a good sensitivity to evaluate soft tissue.12
The aim of the study was to assess joint health of severe hemophilia A children receiving episodic treatment, in Indonesia National Hemophilia Treatment Center, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
METHODS
A cross-sectional study was conducted in the Pediatric Hematology-Oncology Division outpatient clinic of Cipto Mangunkusumo Hospital Jakarta as an Indonesian National Hemophilia Treatment Center in March- September 2015. The protocol of this study has been approved by Medical Ethics Committee of Universitas Indonesia (No. 252/UN2.F1/ ETIK/2015). Written informed consent was obtained from parents of children aged less than 10 years and both parents and child aged >10–18 years. Inclusion criteria were children aged 4–18 years, with severe hemophilia A (factor VIII <1%), history of repeated joint bleeds, and previously received episodic treatment. Exclusion criteria were children with inhibitor factor VIII and/or refusing to participate in the study.
Clinical history
Epidemiological data and clinical history were obtained from the Pediatric Hemophilia Registry and medical record, including age, age of diagnosis, age of first joint bleeding, target joint, number of joint bleedings and factor VIII concentrate received during the last year. Age of diagnosis is the age of the subject when diagnosed as with hemophilia based on factor assay. Age of first joint bleed is the age of the subject when the first joint bleed (one/ more joint/s of the six index joints) occurred. Target joint is defined as a joint in which three or more spontaneous bleeds have occurred within a period of six consecutive months.2 The number of joint bleedings and factor VIII concentrate usage was collected for the last one year. Nutrition status was determined according to body mass index (BMI) calculation of body weight (kg) and body height (m) measurement at the time of HJHS examination and stratified into four groups: underweight (BMI <5th percentile), healthy weight (BMI 5th–<85th percentile), overweight (BMI 85th– <95th percentile), and obese (BMI 95th percentile).
Inhibitor factor VIII
Inhibitor factor VIII was determined using Bethesda assayin hemostasis laboratory of the Department of Clinical Pathology, Cipto Mangunkusumo Hospital at the time of enrollment.
Musculoskeletal assessment
Musculoskeletal assessment was determined by clinical evaluation using HJHS version 2.1 and ultrasonography evaluation using HEAD-US scores of the six index joints (bilateral elbows/ knees/ankles).
HJHS version 2.1 (2011) was performed by trained physiotherapists and medical rehabilitation specialist consultant at the Medical Rehabilitation Department,Cipto Mangunkusumo Hospital. The HJHS version 2.1 (2011) evaluates eight aspects for each joint: severity and duration of joint swelling, muscle atrophy, muscle strength, crepitus in motion, joint pain, joint flexion and extension; with additional global gait assessment at the time of walking, up- and downstairs, running and jumping with one leg. Total scores range from 0–124, a higher score shows more severe joint damage.8
Joint ultrasonography was performed by single radiology specialist consultant from Musculoskeletal Division, Department of Radiology,Cipto Mangunkusumo Hospital according to methodology and scoring system HEAD-US. The HEAD-US method measures joint disease activity by the presence of synovial hypertrophy (minimal/mild-moderate/ severe) and joint damage by looking at the cartilage (normal/cartilage damage <25%/25- 50%/>50%/total damage) and the bone (normal/ mild irregularity of subchondral bone with or without osteophytes/deranged subchondral bone with/without erosions and presence of prominent osteophytes around the joint.11
Data analysis
Data analysis was performed using statistical package for social sciences (SPSS) version 20.0. HJHS and HEAD-US scores were analyzed based on two age groups: 4–10 years and >10–18 years. Descriptive analyses for categorical variables were total and percentage, while for numerical variables were mean and standard deviation (normal distributed data) or median and minimum/ maximum range (abnormal distributed data). Unpaired T-test was used to analyze normal data distribution and Mann–Whitney test was used for abnormal distributed data.
RESULTS
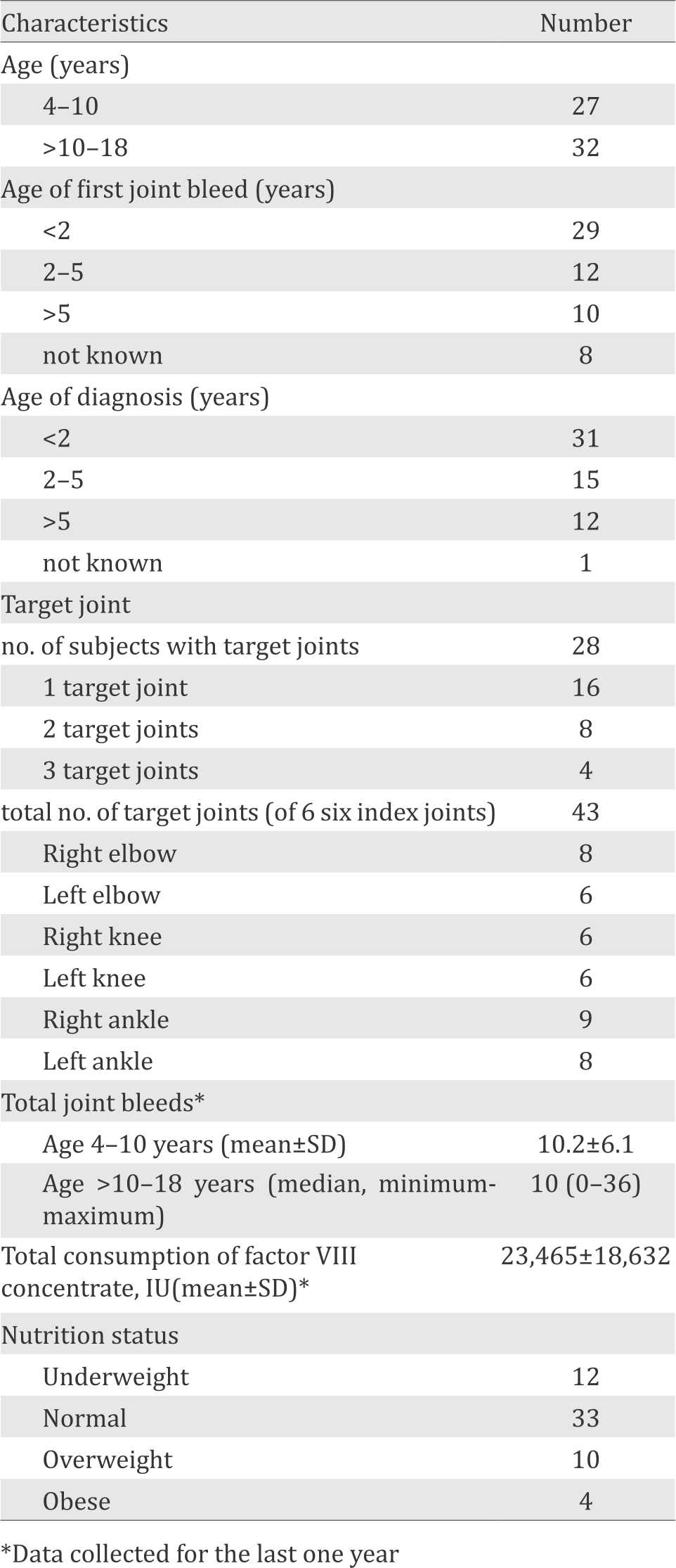
During the study period, fifty-nine subjects were enrolled to the study according to inclusion and exclusion criteria. The age of subjects ranged from 6 to 18 years, with mean of 11.7±3.7 years and median of 11.6 years. Twenty-nine out of 59 (49.2%) subjects experienced first joint bleed before two years of age. Thirty-one out of 59 (52.5%) subjects were diagnosed before two years of age. There were 30 out of 59 (50.85%) subjects with target joints, and the most common target joint was right ankle. All subjects had no previous inhibitor factor VIII. Baseline characteristics of subjects are presented in Table 1.
Table 1. Subject characteristics (n=59)
Mean total HJHS and HEAD-US score in subjects with and without target joints are presented in Table 2. The mean HJHS of subjects aged 4–10 years was 5.7±4.3, with lowest score 0 and highest score 13. In subjects aged >10–18 years, mean HJHS scores was 10.8±9.2 with lowest score 0 and highest score 38. HEAD-US score in subjects aged 4–10 years was 18.7±5.6, lower than in subjects aged >10–18 years (28±7.9, see Table 3).
Table 2. HJHS and HEAD-US scores in subjects with and without target joints
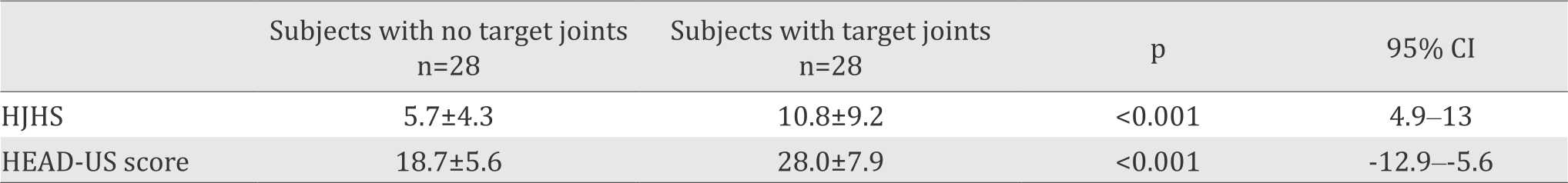
Table 3. HJHS and HEAD-US scores in subjects aged 4–10 years and >10–18 years
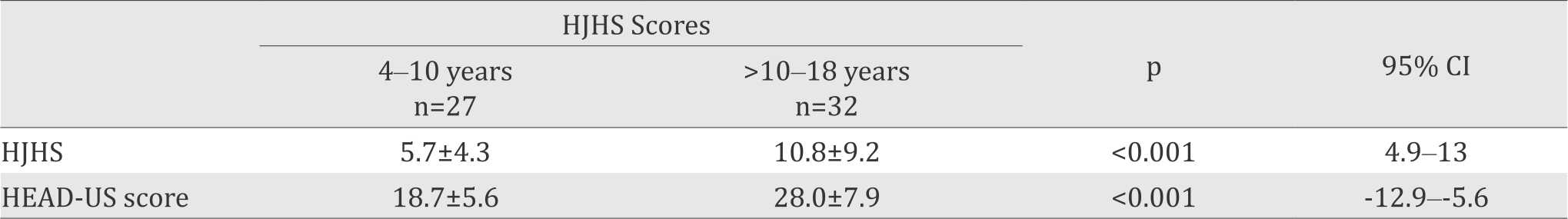
The most affected joints according to HJHS and HEAD-US scores are presented in Figure 1 and Figure 2. All subjects showed negative inhibitor factor VIII. We present the ultrasonography records of each joint (ankle, knee, and elbow) in Figure 3.
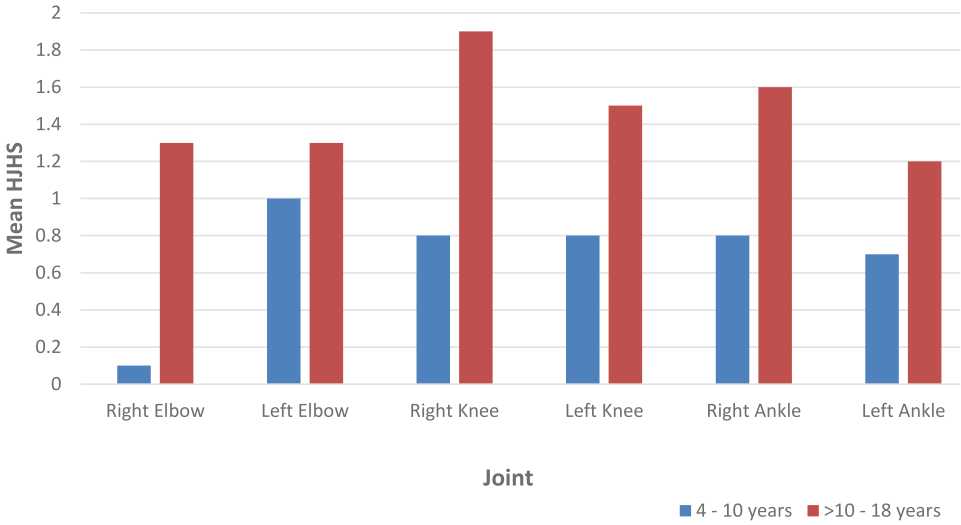
Figure 1. Mean HJHS according to six index joints in subjects aged 4–10 years and >10–18 years
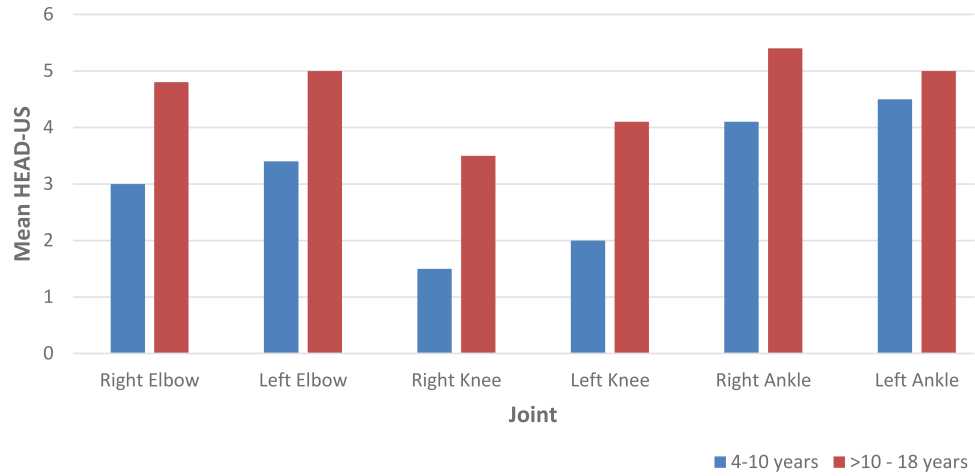
Figure 2. Mean HJHS according to six index joints in subjects aged 4–10 years and >10–18 years
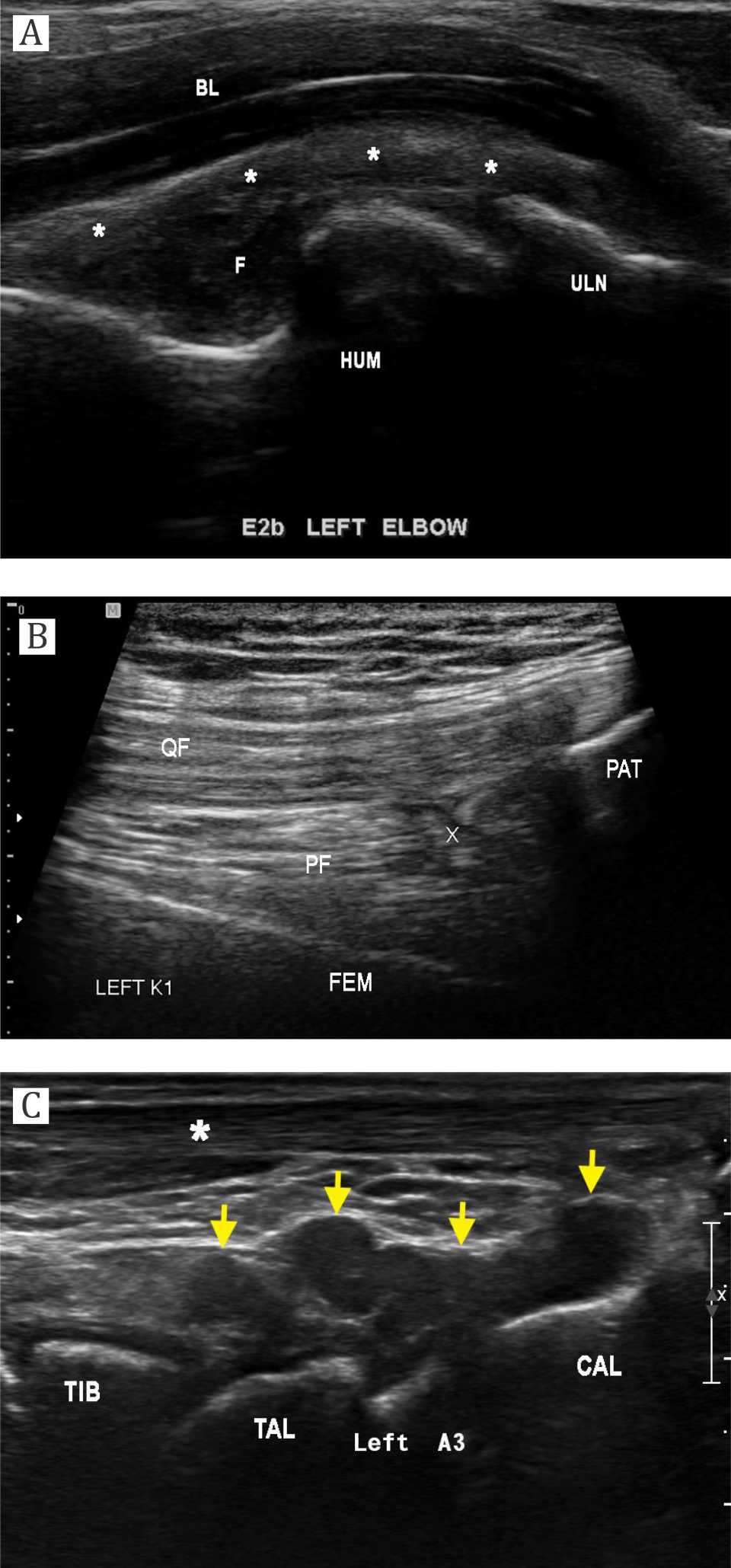
Figure 3. a) Elbow sagittal sonography of a 17-years-old male with severe hemophilia (HEAD-US at E2B site). There is marked iso-hypoechoic synovial hypertrophy (asterisk) that extending to the cubital fossa. Fluid collection was noted within synovial cavity with internal debris (F). Humeral condyle (HUM) shows irregular surface without cartilage layer. Bony irregularity is also seen at the coronoid process of ulna (ULN); b) Knee sagittal sonography of a 15-years-old male with severe hemophilia (HEAD-US at K1 site). There is only small synovial layer (X) identified at the supra patellar region, without any fluid collection. QF= quadriceps femoris tendon; PF= prefemoral fat pad; PAT= patella; FEM= femur; Posterior ankle sagittal sonography of a 9-years-old male with severe hemophilia (HEAD-US at A3 site). There is marked hypoechoic synovial hypertrophy (yellow arrow) extending posteriorly from talotibial and subtalar joint. TIB= tibia; TAL= talus; CAL= calcaneus; asterisk= Achilles tendon
DISCUSSION
Our study assessed musculoskeletal status using the relatively new measurement tools provided by hemophilia experts: HJHS version 2.1 and HEAD-US scores. Both measurement have been shown to be reliable with good sensitivity to detect early joint damage, which we consider the most appropriate available tools for our current situation.
Our study showed that 49.2% of subjects had first joint bleeding before the age of 2 years, while 20.3% subjects between 2–5 years. van Dijk et al1 reported that in 90% of cases with severe hemophilia A, first joint bleeding occurred before the age of 4 years, which is earlier than in mild and moderate hemophilia cases.1 Pollmann et al reported that 44% of severe hemophilia patients experienced their first joint bleed at the age of ≤1 year, while overall mean age of first joint bleed in the study was 1.9 years.13 We found that 10 out of 59 (16.9%) subjects had first joint bleed at the age >5 years, this could be due to milder phenotype of severe hemophilia A caused by specific mutations, although factor VIII activity was <1%. Previous studies reported clinical manifestation variability among severe hemophilia A patients. Aledort et al14 reported that 10–15% of severe hemophilia A patients had milder bleeding phenotype.14
We found 28 out of 59 (47.4%) subjects had target joints, and 10 of them had more than one target joints. The most common target joint was right ankle (9 out of 43 joints), followed by left ankle and right elbow (both 8 out of 43 joints). Bilateral knees and left elbow were the least target joints. Our data showed that all subjects had multiple joint involvements and the most frequently affected joints were ankles.
HJHS was originally designed to detect early joint damage in patients receiving prophylaxis. However, earlier studies showed that this measurement also applicable for episodically treated patients.15,16 Study in Lithuania16 reported HJHS in 20 hemophilia children receiving episodic treatment, mean total scores in patients aged 4–9 years was significantly lower compare to older children aged 10–17 years (11.6 vs 31.5, respectively). Study in Pakistan15 on severe (n=59) and non-severe (n=29) hemophilic patients receiving episodic treatment divided into three developmental age groups (<12 years, 12–16 years, and >16 years), the HJHS and Gilbert scores were significantly lower in the youngest group (p<0.001). Our study showed that HJHS and HEAD-US scores in younger age (4–10 years) were significantly lower than in older subjects (>10–18 years), p<0.001; 95% CI=4.913 and p<0.001; 95% CI= -12.9–5.6, respectively). Our study result is similar with data from several studies that showed a strong correlation between age and HJHS. Moreover, in our study, the result of HJHS was supported by ultrasonography scores. Our results supported that HJHS in children receiving episodic treatment demonstrated progressivity of joint damage with increasing age, especially in countries with limited usage of factor concentrates. These results also showed late manifestations of joint impairment in older haemophilia children treated with episodic treatment.
Comparative study in Lithuania-Denmark17 in patients receiving episodic treatment (n=16) and prophylaxis (n=16) showed better HJHS in prophylaxis group (p<0.001). In the study, subjects were stratified according to age (4–9 years and 10–17 years), and the results also showed better HJHS in younger age group receiving prophylaxis treatment; 2.2 vs 12.5 (p=0.0002) and 3.9 vs36.3 (p<0.0001), respectively. The mean difference of HJHS in younger (4–9 years) compare to older (10–17) years children receiving prophylaxis was not significant, 2.2 vs 3.9 (95% CI= -0.4- 3.9) respectively. The result supported other clinical trials proving that prophylaxis treatment is superior compare to episodic treatment in preventing joint damage progressivity in children with severe hemophilia.18-21 In Indonesia, severe hemophilia chidren receive episodic treatment due to very expensive cost of factor concentrates.
Our study showed that the highest HEAD-US score was found in left ankle in subjects aged 4-10 years, while in subjects aged >10–18 years it was the right ankle. These findings were not corresponding with HJHS results that the most affected joint in subjects aged 4–10 years was left elbow and in subjects aged >10–18 years was right knee. These results may be due to several reasons. Firstly, due to episodic therapy, few subjects may have just recovered from acute hemarthrosis in different intervals prior to HJHS examination that more likely affected HJHS results. We could not wait until two weeks to perform HJHS examination because without prophylaxis we could not predict when the subject will suffer acute hem arthrosis. Secondly, we did not evaluate daily activities between the two agegroups which may affect different target joints.
Our results also showed that HJHS were higher in subjects with target joints (p=0.057; 95% CI=0.0016.9), as well as the HEAD-US scores (p=0.008; 95% CI= -9.8-1.5). These results imply that irreversible joint damage in children treated with episodic therapy is corresponding with joint function evaluated by HJHS. We did not analyze correlation between HJHS and HEAD-US due to insufficient sample size.
Recent observational study reported by Altisent et al22 showed the utilization of HJHS and HEADUS scores in severe hemophilia children aged 4–19 years (n=25) treated with prophylaxis treatment and no history of inhibitor. The median of prophylaxis dose was 65.4 IU/kg/week. The annual joint bleeding rate was 0.2, while the median total HJHS was 0 (range 0–13) and total HEADUS 1 (0–8). Ultrasound examination detected minimal changes in 19.6% of joints with normal physical function, on the contrary 12.2% of joints with normal ultrasound results showed changes in HJHS results. The authors concluded that ultrasonography with HEAD-US method detected a higher percentage of abnormalities than the physical evaluation, however clinical implications of these findings still need to be determined in larger prospective cohort. In the study, total HJHS scores correlated with age (r=0.56, p=0.006), weight (r=0.61, p=0.002), and age at the start of prophylaxis (r=0.63; p=0.001).22 In our study, the HJHS and HEAD-US scores were higher compared to Altisent’s study22 due to different treatment regimen, showing that prophylaxis can preserve joint function and prevent joint damage in children with severe hemophilia A.
Our study has several limitations. This was the first time HJHS and HEAD-US procedures were applied to assess joint status in our hospital. HJHS has demonstrated good construct validity and internal validity, but has not been used as routine evaluation in our center. The HEAD-US is a novel ultrasound technique that needs to be validated in large series of patients. There is also a possibility of different daily activity among subjects that we did not evaluate. Retrospective data of number of joint bleedings and factor VIII used for episodic treatment in our study together with a cross-sectional evaluation of joint status needs to be confirmed with prospective studies.
In conclusion, we found that joint outcome of severe hemophilia A children receiving episodic treatment in our center evaluated by HJHS and ultrasonography showed more severe damage in older (aged >10–18 years) compare to younger (aged 4–10 years) children. It is important to perform routine evaluation of joint status to monitor progressivity of joint damage over time, so treatment adjustment can be considered. Episodic treatment has been proven to show poor joint outcome in children with severe hemophilia, however there are barriers for implementing prophylaxis treatment in developing countries as Indonesia.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
We would like to thank our study coordinators dr. Mega Utami and dr. Randi R. Mulyadi and our data manager Dina Watanabe, SKM. We also would like to thank physiotherapist team who performed hemophilia joint health score evaluation (Sri N. Fauza, SST.FT, Pipin Suparmi, SST.FT, Titik Manik, SMPh, Sofia Resti H, S.FT, Abdul Jamil, SST.FT and Safarudin, Amd.FT). This study was supported by research grant from Universitas Indonesia (Multidiscipline Research Grant No. 1633/UN2. R12/HKP.05.00/2015).
REFERENCES
- van Dijk K, van der Bom JG, Fischer K, Grobbee DE, van den Berg HM. Variability in clinical phenotype of severe haemophilia: The role of the first joint bleed. Haemophilia. 2005;11:438–43
- Srivastava A, Brewer AK, Mauser-bunschoten EP, Key NS, Kitchen S. Treatment Guidelines Working Group on Behalf of The World Federation of Hemophilia: Guidelines for the management of hemophilia. Haemophilia. 2013;19:1–47.
- Perhimpunan Hematologi dan Transfusi Darah Indonesia (PHTDI) & Himpunan Masyarakat Hemofilia Indonesia (HMHI). Panduan diagnosis dan tata laksana hemofilia. 1 ed: Jakarta: PHTDI & HMHI; 2013. hal. 1‒31.
- Acharya SS, Kaplan RN, Macdonald D, Fabiyi OT, DiMichele D, Lyden D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 2011;117:2484–93.
- Handryastuti, Djajadiman G, Arwin AP Akib. Clinical characteristics of hemophilia A patients with hemarthrosis. Paediatr Indones. 2002;42:131–7.
- Hilliard P, Funk S, Zourikian N, Bergstrom BM, Bradley CS, McLimont M, et al. Hemophilia Joint Health Score reliability study. Haemophilia. 2006;12:518–25.
- Feldman BM, Funk S, Lundin B, Doria AS, Ljung R, Blanchette V, et al. Musculoskeletal measurement tools from the International Prophylaxis Study Group (IPSG). Haemophilia. 2008;14:162–9.
- International Prophylaxis Study Group. Physical Therapy Expert Working Group. Hemophilia Joint Health Score. 2011:1–31.
- Sierra Aisa C, Lucia Cuesta JF, Rubio Martinez A, Fernandez Mosteirin N, Iborra Munoz A, Abio Calvete M, et al. Comparison of ultrasound and magnetic resonance imaging for diagnosis and follow-up of joint lesions in patients with haemophilia. Haemophilia. 2014;20:51–7.
- Querol F, Rodriguez-Merchan EC. The role of ultrasonography in the diagnosis of the musculoskeletal problems of haemophilia. Haemophilia. 2012;18:e215‒26.
- Martinoli Carlo, Alberighi Ornella Della Casa, Di Minno Giovanni, Graziano Ermelinda, Molinari Angelo Claudio, Pasta Gianluigi, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US). J Thromb Haemost. 2013;109:1170–9.
- Muca-Perja M, Riva S, Grochowska B, Mangiafico L, Mago D, Gringeri A. Ultrasonography of haemophilic arthropathy. Haemophilia. 2012;18:364–8.
- Pollmann H, Richter H, Ringkamp H, H J. When are children diagnosed as having severe haemophilia and when do they start to bleed? A 10-year single-centre PUP study. Eur J Pediatr. 1999;158:S166–70.
- Aledort LM, Haschmeyer RH, Petterssont H, & the Orthopaedic Outcome Study Group. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. J lnt Med. 1994;236:391‒9.
- Khanum F, Bowen DJ, Kerr BC, Collins PW. Joint health scores in haemophilia A cohort from Pakistan with minimal or no access to factor VIII concentrate : correlation with thrombin generation and underlying mutation. Haemophilia. 2014;20:426‒34.
- Trakymiene SS, Ingerslev J, Rageliene L. Utility of the Haemophilia Joint Health Score in study of episodically treated boys with severe haemophilia A and B in Lithuania. Haemophilia. 2010;16:479–86.
- Trakymiene SS, Clausen N, Poulsen LH, Ingerslev J, Rageliene L. Progression of haemophilic arthropathy in children: a Lithuanian - Danish comparative study. Haemophilia. 2013;19:212–18.
- Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM, and The ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9:700–10.
- Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44.
- Verma SP, Dutta TK, Mahadevan S, Nalini P, Basu D, Biswal N, et al. A randomized study of very low-dose factor VIII prophylaxis in severe haemophilia - A success story from a resource limited country. Haemophilia. 2016;22:342‒8.
- Wu R, Luke KH, Poon MC, Wu X, Zhang N, Zhao L, et al. Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: a pilot study in China. Haemophilia. 2011;17:70–4.
- Altisent C, Martorell M, Crespo A, Casas L, Torrents C, Parra R. Early prophylaxis in children with severe haemophilia A : clinical and ultrasound imaging outcomes. Haemophilia. 2015;22:218–24.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id