
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Stable archaeal tetraether lipid liposomes for photodynamic application: transfer of carboxyfluorescein to cultured T84 tumor cells
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i4.1506 Med J Indones. 2016;25:196–206
Received: July 20, 2016
Accepted: September 25, 2016
Author affiliation:
1 Faculty of Medicine, Johann-Wolfgang-Goethe University, Frankfurt am Main, Germany
2 Center of Urology, Asklepios Paulinen Klinik Wiesbaden, Germany
3 Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok, Indonesia
4 Medical Research Unit, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Hans-Joachim Freisleben
E-mail: hj.freisleben@t-online.de
Background
Archaeal membranes have phytanyl ether lipids instead of common fatty acid-glycerol esters in bacterial and eukaryotic cells. Sulfolobus and Thermoplasma species have unique membrane-spanning tetraether lipids (TEL), which form stable liposomes. Recently, we cultured Thermoplasma species from the Indonesian volcano Tangkuban Perahu and isolated TEL. The purpose of this in vitro study is to investigate the transfer of fluorescent dye from stable TEL liposomes to cultured colon carcinoma cells.
Methods
TEL was extracted from cultured cells with chloroform-methanol (1:1), then it was fractionated and purified via diethylaminoethyl-cellulose-acetate columns and activated charcoal for the formation of stable liposomes. For the fluorescence exchange assay, TEL liposomes were loaded with water-soluble carboxyfluorescein (CF). Staining experiments were conducted with various cell cultures, and T84 colon carcinoma cells were chosen for the main experiments. Liposome stability was tested by light scattering and electron microscopic size determinations as well as by unspecific CF release at low pH (6.0–7.4) and increased temperature (4–50°C/70°C).
Results
TEL liposomes exhibit high stability and extremely low proton permeability at low pH. CF staining of cultured T84 colon carcinoma cells appeares more intensive from TEL liposomes than from dipalmitoylphosphatidylcholine liposomes.
Conclusion
The results of this in vitro study demonstrate CF staining of colon carcinoma cells and high stability of TEL liposomes at low pH, matching the condition in the gastro-intestinal (GI) route and in the urogentital (UG) tract. For this reason, in vivo studies on liposomal fluorescent photosensitizers for topical application of photodynamic cancer therapy in the GI and UG tracts should be carried out.
Keywords
archaea, archaeosomes, carboxyfluorescein, liposomes, T84 colon carcinoma cells, tetraether lipid, Thermoplasma
Recently, we reported about the isolation of Sufolobus species1 and the culture and identification of Thermoplasma acidophilum and Thermoplasma volcanium from Tangkuban Perahu.2 Sulfolobales and Thermoplasmatales produce high amounts of unique tetraether lipids to build their cell membranes.3 Whereas Sulfolobales have a cell wall, Thermoplasmatales do not; their lipid membrane is naked and easily accessible. Therefore, we especially focus on Thermoplasma species because their membrane-spanning tetraether lipids (TEL) form stable liposomes.4 In the laboratory, heterotrophic growth optimum of Th. acidophilum strain DSM 1728 (DSM, Deutsche Sammlung von Mikroorganismen, Leibniz Institute DSMZGerman Collection of Microorganisms and Cell Cultures) is at pH 2 and 59°C in a sulfuric acidcontaining Freundt’s medium.5 During the last 20 years, archaeal tetraether lipid technology was transferred from Frankfurt am Main, Germany by one of us to Indonesia. We isolated TEL from these cultures and showed the same lipid pattern as in Th. acidophilum strain DSM 1728 and purified the main polar glycophospholipid (MPL) both in our laboratories in Germany and in Indonesia.6 Research on the biomedical application of TEL liposomes have been started 20 years ago in Germany and is now continued by our working group in Indonesian laboratories. Experimental laboratory data were also confirmed by our computerized simulation of a tetraether lipid membrane model.7 Because of the chemical stability of tetraether lipids, we are especially interested in the application of stable liposomes of this lipid in the gastrointestinal (GI) and urogenital (UG) tracts. The purpose of this study is to examine the transfer of fluorescent dyes to carcinoma cells. This is particularly suitable to topical tumor treatment in the GI tract via oral administration and in the UG tract via trans-urethral cystoscopic administration. More specifically, we want to find out whether TEL liposomes can be used for liposomal photodynamic diagnosis (PDD) and therapy (PDT), which have attracted great attention during the last 10 years. The benefits of 5-aminolevulinic acid (5-ALA) PDD in colorectal oncology – including abdominal metastases – have been reported by Kondo et al.8 Furthermore, 5-Ala PDD is widely performed in urological oncology with transurethral fluorescence cystoscopy, especially for the detection of small bladder tumors9 and urothelial premalignant lesions,10 as well as in prostatectomy.11 Whereas the endogenous metabolic pathway supports 5-ALA PDD and PDT,12 the way of administration of 5-ALA to the target tissue still needs improvement; liposomal delivery in the GI or UG tracts could provide solutions. In urology our study aims to the intravesical application with acid-stable archaeosomes via trans-urethral cystoscopic equipment.
METHODS
Materials, instruments, and human cell cultures
Phosphatidylcholine (PCL)=dipalmitoylphosphatidylcholine (DPPC), carboxyfluorescein (CF), Nile red, and Triton X-100 were purchased from Sigma (Deisenhofen, Germany); bromobimanes from Calbiochem, Bad Soden, Germany; Sephadex G-50 fine from Pharmacia, Freiburg, Germany; all other substances, solutions and buffers were obtained from Merck, Darmstadt, Germany or from their subsidiaries in Jakarta or Singapore.
Two different types of fluorometers were used in our investigations: a filter fluorometer model 112, Sequoia Turner Corp. California, USA with excitation filter 7.60 (λ=315-385 nm) and emission filter 2A (λ>415 nm) and a spectrofluorometer RF-5001 PC, Shimadzu Corp. Darmstadt, Germany. The two inverse phase contrast fluorescence microscopes used in this study were Type BH2-RFC-1, Olympus Optical Co. Hamburg, Germany and from Olympus subsidiary in Jakarta, Indonesia.
Human T84 colon carcinoma cells were kindly provided by the Center of Physiology, JHW Goethe-University of Frankfurt/Main, Germany and grown at 37°C under an atmosphere containing 5% CO2 in Nunc Lab-TekTM culture discs (Nunc, Wiesbaden, Germany). Culture medium consisted of 500 mL of Dulbecco’s Modified Eagle Medium containing NaHCO3 1.85 g; HEPES 3.9045 g; penicillin (10,000 U) / streptomycin (10,000 μg/mL); Ham’s F12 5.3 g; fetal calf serum (FCS) 5%. Nunc Lab-Tek discs can be directly used as microscope slides for adherent cell colonies.
Thermoplasma TEL extraction, purification and physical characterization
Thermoplasma species were cultured according to Freisleben et al5 or Malik et al.2 Extraction of the TEL fractions and purification of the main phospho-glyco-tetraether lipid (MPL) were accomplished according to Antonopoulos et al.6
Preparation of liposomes and archaeosomes
Archaeosomes, tetraether lipid (TEL) and DPPC (PCL) liposomes were generally (if not indicated differently) prepared according to the classical methods of liposome technology as compiled in New.13 The polar lipid fraction or purified tetraether lipid was dissolved in organic solvent (chloroform-methanol mixture), which was subsequently removed by evaporation in a rotavapor (Büchi, Switzerland) at 45°C. For liposome or archaeosome preparation, the dry lipid film was suspended in phosphate-buffered saline (PBS) and was shaken by hand at room temperature (RT) to disperse the lipid film until a homogenous multilamellar vesicle (LMV) suspension was formed with a lipid concentration of 20 mg/mL. For carboxyfluorescein (CF)-stained liposomes, carboxyfluorescein (CF) was added to PBS at 20 μM concentration. Hand-shaken liposomes or archaeosomes were sonicated with a Branson Sonifier at 20 kHz for five minutes on ice before applied to filter extrusion by means of a LiposoFast™ Extruder using 100 nm poresized polycarbonate filters (Avestin Inc., Ottawa, Canada). After preparation of the liposomes, nonliposomal material was removed through open column chromatography utilizing Sephadex G 50 fine (Pharmacia, Freiburg, Germany).
Although TEL liposomes are heat stable and can be autoclaved or even heat sterilized,14 we used sterile filtration, especially, since the latter can be applied equally to any other lipids (in our case PCL, DPPC) and can be done in one run with the extrusion method for liposome preparation if polycarbonate filters with 100 nm pores are used. The influence of protons, temperature, alcohols and detergents on swelling and long-term shelf stability of the liposomes was determined according to Freisleben.3,4
Intermembrane exchange assay
In order to test the release of lipophilic compounds to membranes of intact target cells from TEL liposomes intermembrane exchange assay to erythrocyte ghosts was carried out.15,16 Nile red or MBB was mixed with TEL at 20 μM concentration as described in 2.3 and co-evaporated. Lipid film formation incorporated the lipophilic dyes almost quantitatively at the low concentrations was used for our experiments. Liposomes were then prepared as described and passed through Sephadex G50 columns to remove non-entrapped dyes. Control experiments for the demonstration of membrane fusion between liposomes and erythrocyte ghosts were carried out by the incorporation of cholesteroyl-anthracen-9-carboxylate (CA9C) into erythrocyte ghosts and of dihexadecylamino-7-nitrobenzo-2-oxa-1,3-diazole (DH-NBD) into the liposomal membrane.
RESULTS
Physicochemical characterization of liposomes
Our preparation method yielded uniform TEL liposomes with narrow size distribution of 150.0±22.1 nm. Besides the method of preparation, the grade of purity of the lipid was important; particle size decreased in parallel with higher purity of tetraether lipid, primarily with the removal of pigments and lipopolysaccharide complexes (not shown). The zeta potential of TEL liposomes was -58.2±0.2mV at pH 7.4 in diluted PBS as determined in a Malvern Zeta Sizer. For comparison, DPPC liposomes were slightly smaller (143.5±41.0 nm) than TEL liposomes.
TEL liposomes were stable for more than 100 weeks when stored in PBS (10 mg lipid/mL) at 37°C, RT, and at 4–8°C, as measured by changes in size distribution (swelling). For comparison, liposomes from DPPC were stable only up to four weeks in the refrigerator (4–8°C), at RT and 37°C only for a few days. At high temperatures (>60°C), TEL liposomes were stable for 10 weeks.
Swelling of TEL liposomes by addition of glycerol was less than 5% at RT. The liposomes also resisted several-fold higher detergent and alcohol concentrations than DPPC liposomes. Proton leakiness of common liposomal membranes was about 50-times higher than that of TEL membranes.
Loading of liposomes (incorporation of lipophilic or hydrophilic compounds)
Most of CF was found dissolved in the outer volume and some of it was attached to the outer surface of the liposomes. Non-liposomal CF (i.e., not associated with, entrapped or attached to liposomes) was removed by open column separation with Sepadex G 50 fine (principle of “Penefsky” columns). On the other hand, the experimental incorporation of lipid-soluble compounds, i.e. Nile red and thiolyte MBB into liposomes of archaeal lipids showed that they were almost quantitatively taken up into the liposomal membrane.
The incorporation rates of water-soluble compounds, such as CF can be explained as follows. Hydrophilic CF is dissolved in the buffer, entrapped into the liposomes when they form closed spherical particles, and thus distributed between the inner volume of the liposomal lumen and the outer volume. The amount of lipid was 20 mg/mL and the outer volume exceeded the inner volume. Hence, most of CF was not entrapped and subsequently removed by open column chromatography. The remaining liposomal CF was sufficient for the staining experiments. Lipophilic compounds (Nile red or thiolyte MBB) were taken up into the liposomal membrane by approximately 100%.
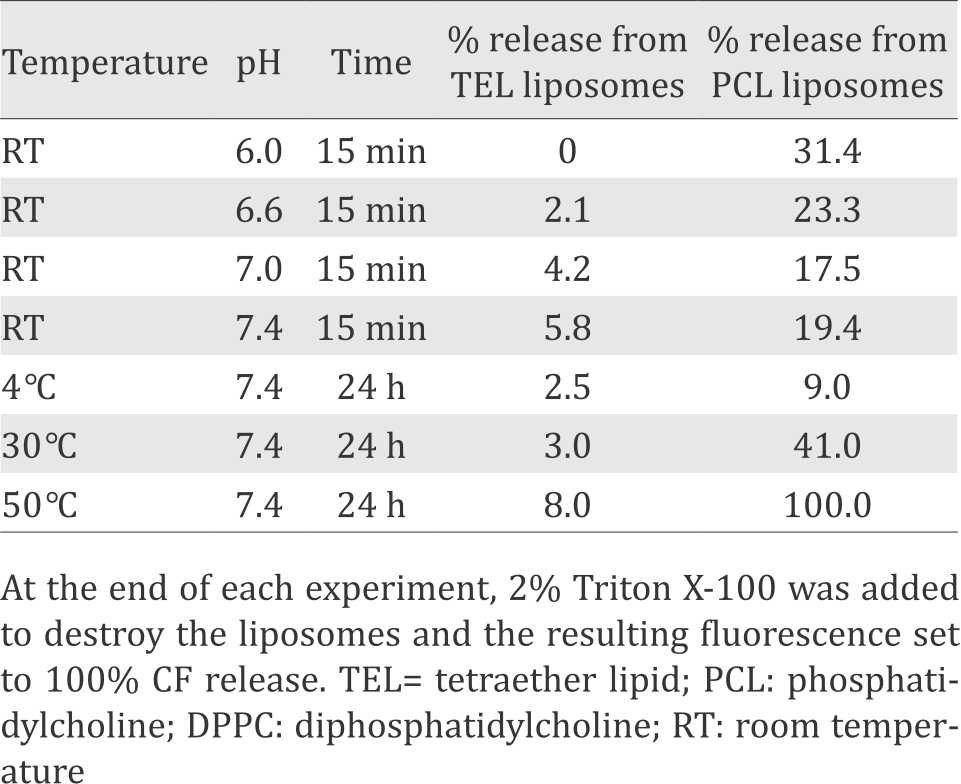
Table 1 at the end of each experiment, 2% Triton X-100 was added to destroy the liposomes and the resulting fluorescence set to 100% CF release; TEL, tetraether lipid; PCL, phosphatidylcholine = DPPC, diphosphatidylcholine.
Table 1. Control experiments: comparison of non-specific CF release (%)
Preliminary experiments: interaction with cell membranes and cells
The study for CF staining from liposomal TEL was performed by using various cell cultures available in the laboratories of the Medical Faculty in Frankfurt, e.g., human colon carcinoma HT-29 and T84 cell lines, human hepatocellular carcinoma cells (HepG2), cystic fibrosis human ductal pancreatic adenocarcinoma cells (CFpac), Chinese hamster ovary cells (CHO), baby hamster kidney cells (BHK), and primary normal human dermal fibroblasts (NHDF). At liposomal lipid concentration of 200 μg/mL in the medium, cell-bound fluorescence was detected at gradual intensity in the sequence: T84>HT29> HepG2=CHO>CFpac>NHDF>BHK. The observed differences in staining experiments depended on the amount of liposomal lipid applied in the experiments; doubling the lipid concentration to 400 μg/mL modified the fluorescence intensity to CHO>>HepG2≥HT-29>>CFpac (not shown).
The interaction with isolated red cell membranes (erythrocyte ghosts) showed that Nile red and MBB were taken up quantitatively into the erythrocyte membranes by intermembrane exchange within 60 minutes. Membrane fusion between liposomal TEL membrane and erythrocyte membrane could not be proven.
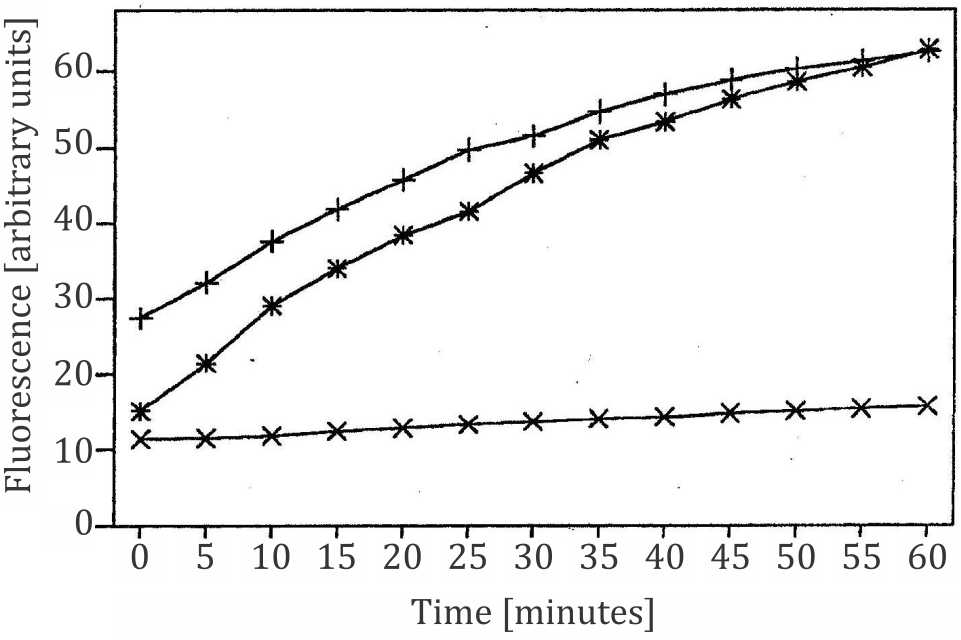
Figure 1. Transmembrane exchange of monobromobimane (MBB) from TEL liposomes to isolated erythrocyte membranes (ghosts); x= negative control, fluorescence of TEL liposomes labeled with MBB, no ghosts present (excitation at 380 nm, emission at 480 nm); += positive control, ghosts labeled with MBB, no liposomes present; the time course of increasing fluorescence emission denotes the reaction kinetics between MBB and sulfhydryl groups in the ghosts; *= TEL liposomes labeled with MBB in the presence of ghosts; the time course denotes the intermembrane exchange of MBB from liposomes to the erythrocyte membrane and its reaction with sulfhydryl groups in the cell membrane. Ghosts were incubated with 20 μM concentration of MBB for 2 minutes before measurement of fluorescence was started. Liposomes with MBB were passed through a Sephadex column before added to ghosts
As demonstrated in this experiment lipophilic dyes (e.g., MBB and Nile red) are transferred from liposomes to cell membranes via intermembrane exchange. The comparable mechanism with hydrophilic dyes like CF is called contact release, i.e., release of CF from liposomes and uptake into cells if loaded liposomes touch the cell membrane or are attached to it.15
Our experiments with erythrocyte ghosts containing fluorescent dye cholesteroylanthracene- 9-carboxylate (CA9C) and TEL liposomes containing dihexadecylamino-7- nitrobenzo-2-oxa-1,3-diazole (DH-NBD) showed that almost no fusion between liposomal and cell membranes occurred, i.e., excitation at λ 370 nm only slightly increased emission at 550 nm and decreased emission at 450 nm (not shown) through direct interaction and energy transfer between CA9C and DH-NBD.13 Although membrane fusion and uptake of liposomes into cultured T84 tumor cells may occur, our experiments confirmed that intermembrane exchange is possible without membrane fusion and/or the uptake of liposomes into cells.16 Liposome–cell interactions and uptake into tumor cells were investigated, and effects of the liposomal protein corona extensively discussed.17 The latter phenomenon depends on the environment surrounding the liposomes and on the formation of a biomolecular corona at the liposomal surface, which differs between cell cultures and the biological systems in the body (e.g., GI or UG tracts or blood circulation). In general, formation of a biomolecular corona at the liposomal surface appears to enhance uptake into tumor cells.17 These phenomena will be investigated with our TEL liposomes in the future.
As a first approach, incorporation and release of CF without the presence of cell membranes or intact cells was studied with sonicated TEL liposomes and compared to liposomes made of PCL (DPPC) and egg yolk lecithin (EYL). At the end of each experiment, 2% Triton X-100 were added for 100% release of CF. Temperature- and pH-dependent release was 5–10 times lower from TEL than from common liposomes with greatest differences at low pH and high temperature (Table 1). The concentrations of alcohols and detergents necessary for 50% CF release were considerably higher with TEL than with common liposomes. These experiments demonstrate that the leakiness of common PCL (DPPC) liposomes is considerably higher than that of TEL liposomes or archaeosomes.3
Main experiments: interaction with cultured T84 colon carcinoma cells
From the orientation in our preliminary experiments, we used the liposomal lipid concentration of 200 μg/mL in the main experiments of cellular CF staining. Now, we report on the promising interaction with T84 colon carcinoma cells. Cell cultures were incubated up to four hours. After more than four hours incubation, fluorescence became diffuse, seemed less associated with cells and differences between cell cultures blurred.
Cultured T84 colon carcinoma cells were incubated with CF (Figure 2A,B) or liposomal CF (Figures 2C,D through 5), either after trypsination or as adherent colonies. The latter experiments were conducted in order to rule out mere effects of trypsination on our results. Figure 2A,B shows control experiments with trypsinated T84 cells incubated for 30 minutes with non-liposomal CF. No cell-bound fluorescence can be seen. Figure 2C,D shows control experiments with CFloaded DPPC (PCL) liposomes immediately after addition, i.e., without incubation (incubation time= 0). Almost no cell-bound fluorescence can be seen. Figure 3A,B shows T84 cell colonies incubated with CF-loaded DPPC (PCL) liposomes for one hour. Figure 3A is the picture in the phase contrast microscope and 3B is the fluorescence picture with weak diffuse CF staining. Figure 3C,D shows T84 cell colonies incubated with CF-loaded TEL for one hour. Figure 3C is the phase contrast picture, and figure 3D is the fluorescence picture with weak CF staining.
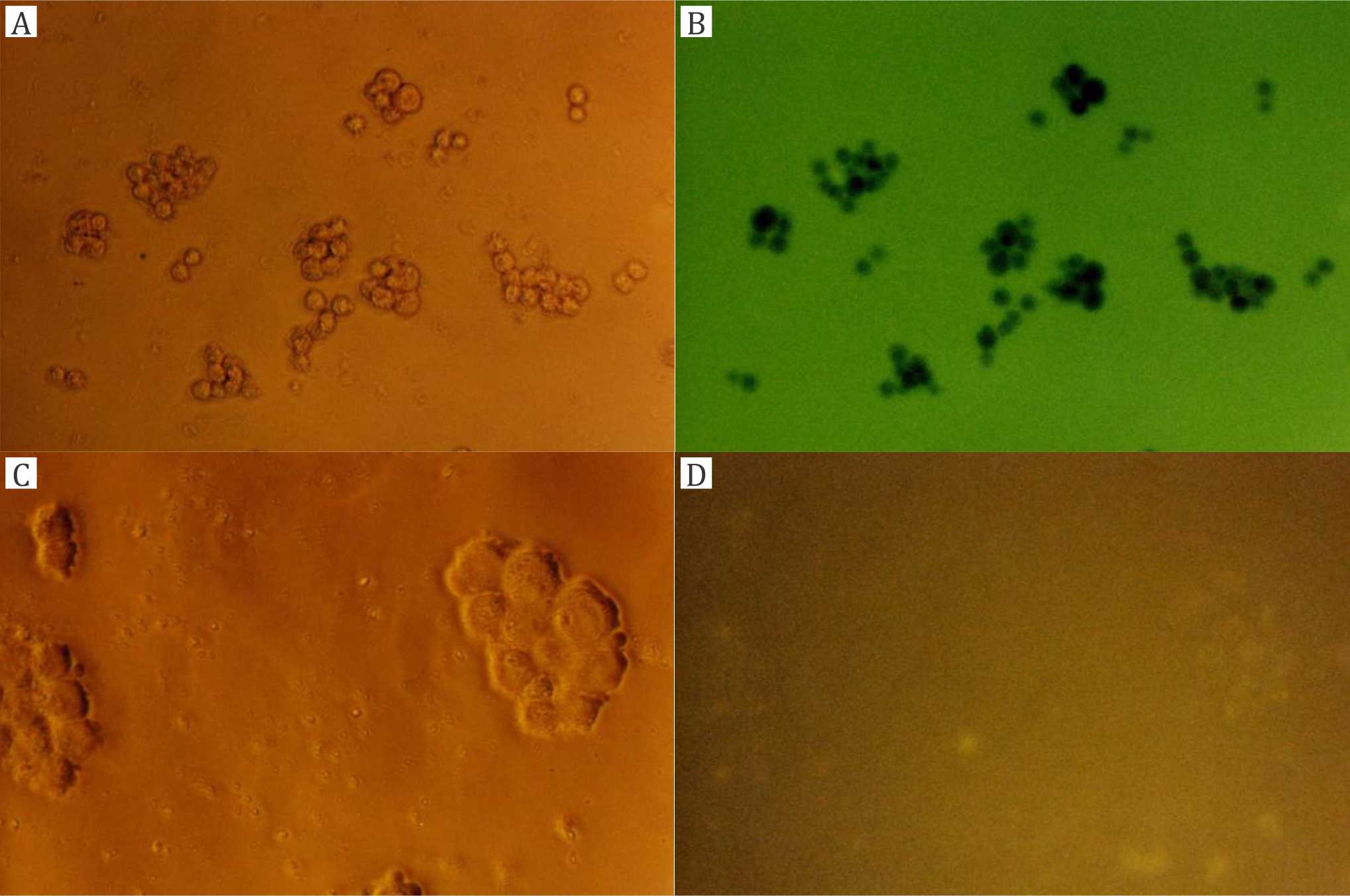
Figure 2. Control experiments with trypsinated T84 cells: A, B) treated with carboxyfluorescein (CF, incubation 30 minutes without liposomes, magnification 200x); C, D) treated with CF-loaded dipalmitoylphosphatidylcholine (DPPC) liposomes (no incubation time, magnification 400x); A, C) phase contrast microscope; B, D) fluorescence microscope
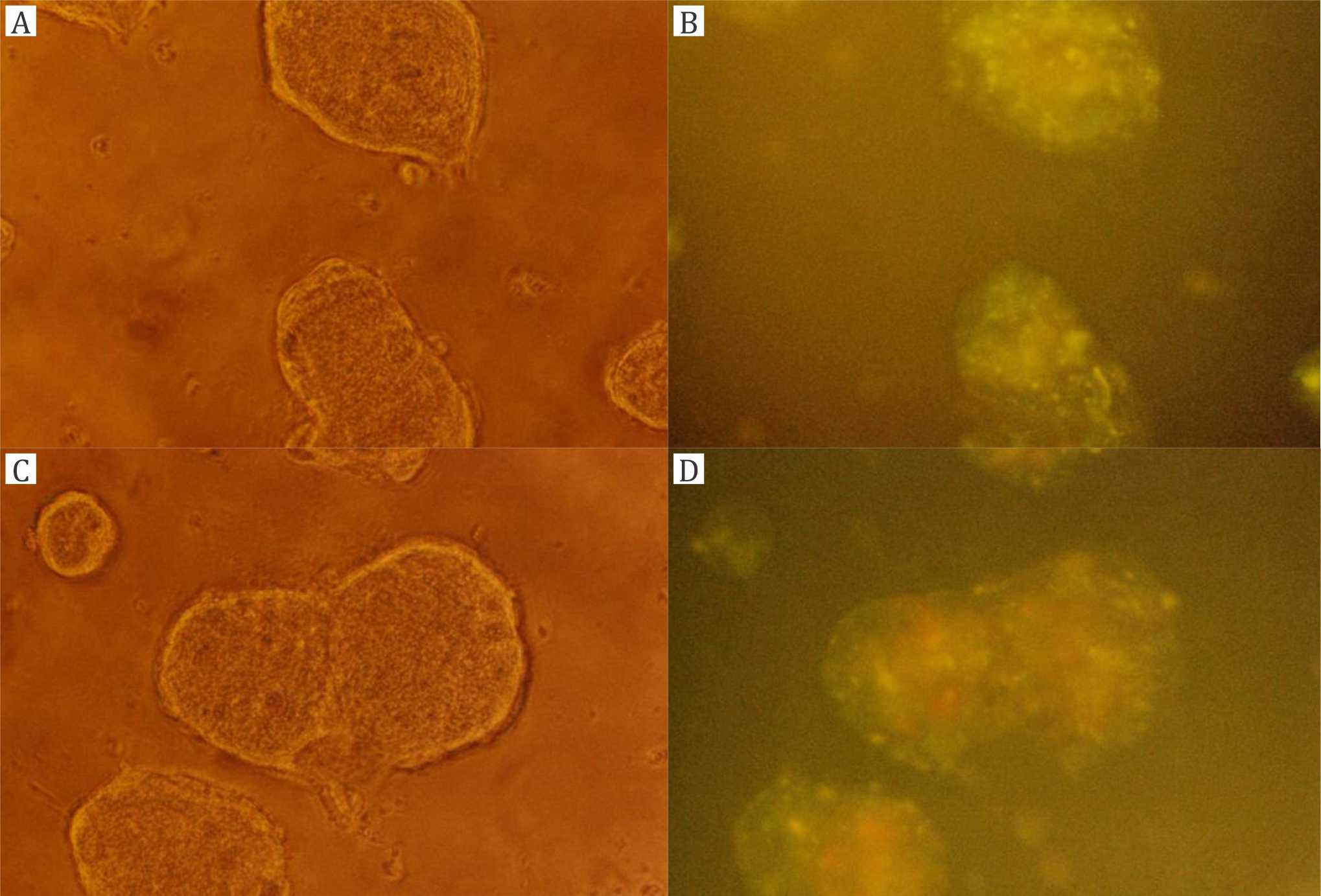
Figure 3. Experiments with T84 cell colonies (magnification 200x): A, B) incubation with carboxyfluorescein (CF)-loaded dipalmitoylphosphatidylcholine (DPPC) liposomes for one hour; C, D) incubation with CF-loaded tetraether lipid (TEL) liposomes for one hour; A, C) phase contrast microscope; B, D) fluorescence microscope
Figure 4A,B shows trypsinated T84 cells incubated with CF-loaded DPPC (PCL) liposomes for one hour. Figure 4A is the phase contrast picture, and figure 4B is the fluorescence picture with slightly stronger CF staining than in figure 3B. Figure 4C,D shows trypsinated T84 cells incubated with CFloaded TEL liposomes for two hours. Figure 4C is the phase contrast picture, and figure 4D is the fluorescence picture with very strong CF staining.
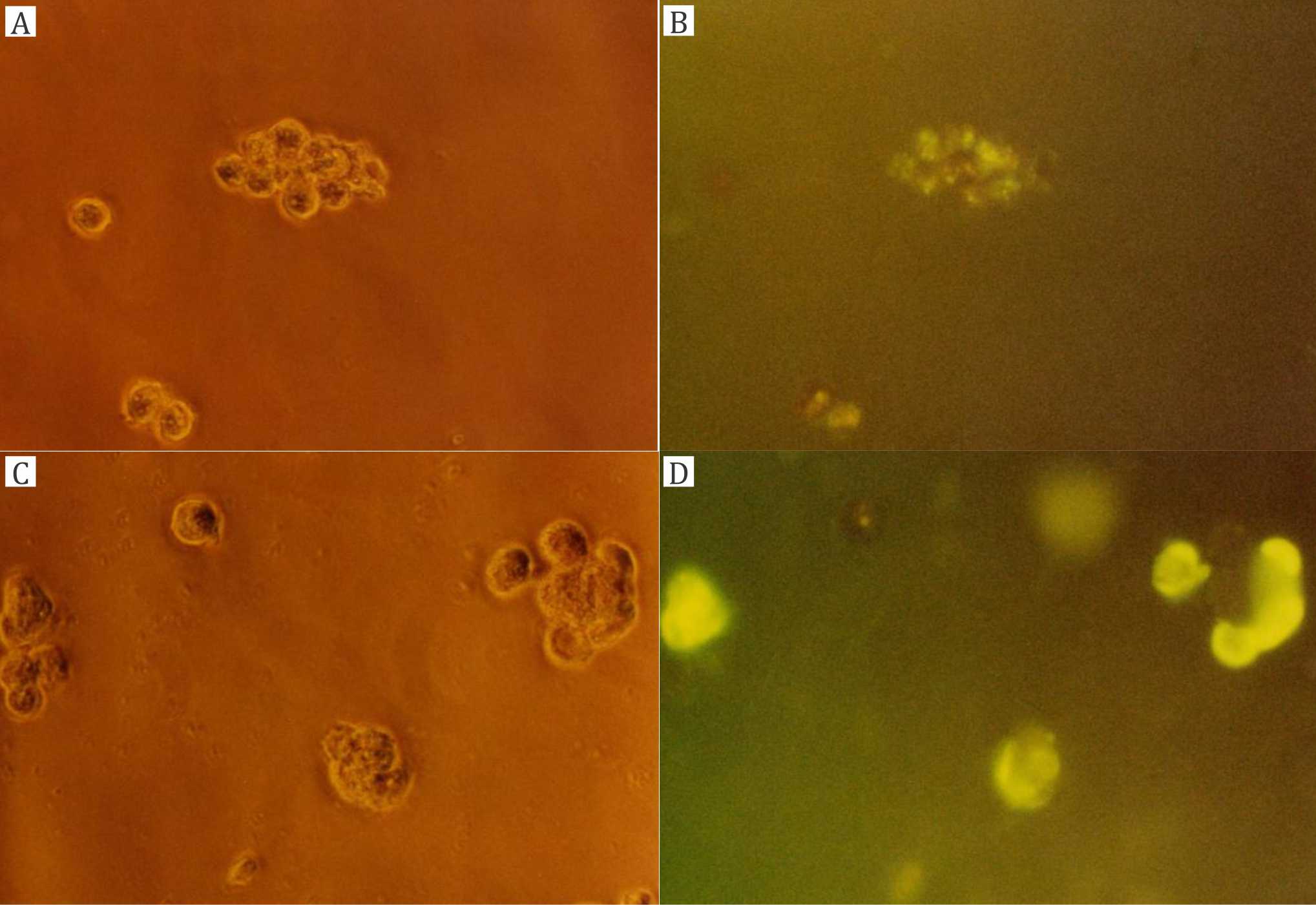
Figure 4. Experiments with trypsinated T84 cells (magnification 400x): A, B) incubation with carboxyfluorescein (CF)-loaded dipalmitoylphosphatidylcholine (DPPC) liposomes for one hour; C, D) incubation with CF-loaded tetraether lipid (TEL) liposomes for two hours; A, C) phase contrast microscope; B, D) fluorescence microscope
Figure 5A,B shows T84 cell colonies incubated with CF-loaded DPPC (PCL) liposomes for four hours. Figure 5A is the phase contrast picture, and figure 5B is the fluorescence picture with diffuse CF staining. Figure 5C,D shows T84 cell colonies incubated with CF-loaded TEL liposomes for two hours. Figure 5C is the phase contrast picture, and figure 5D is the fluorescence picture with strong and clear CF staining.
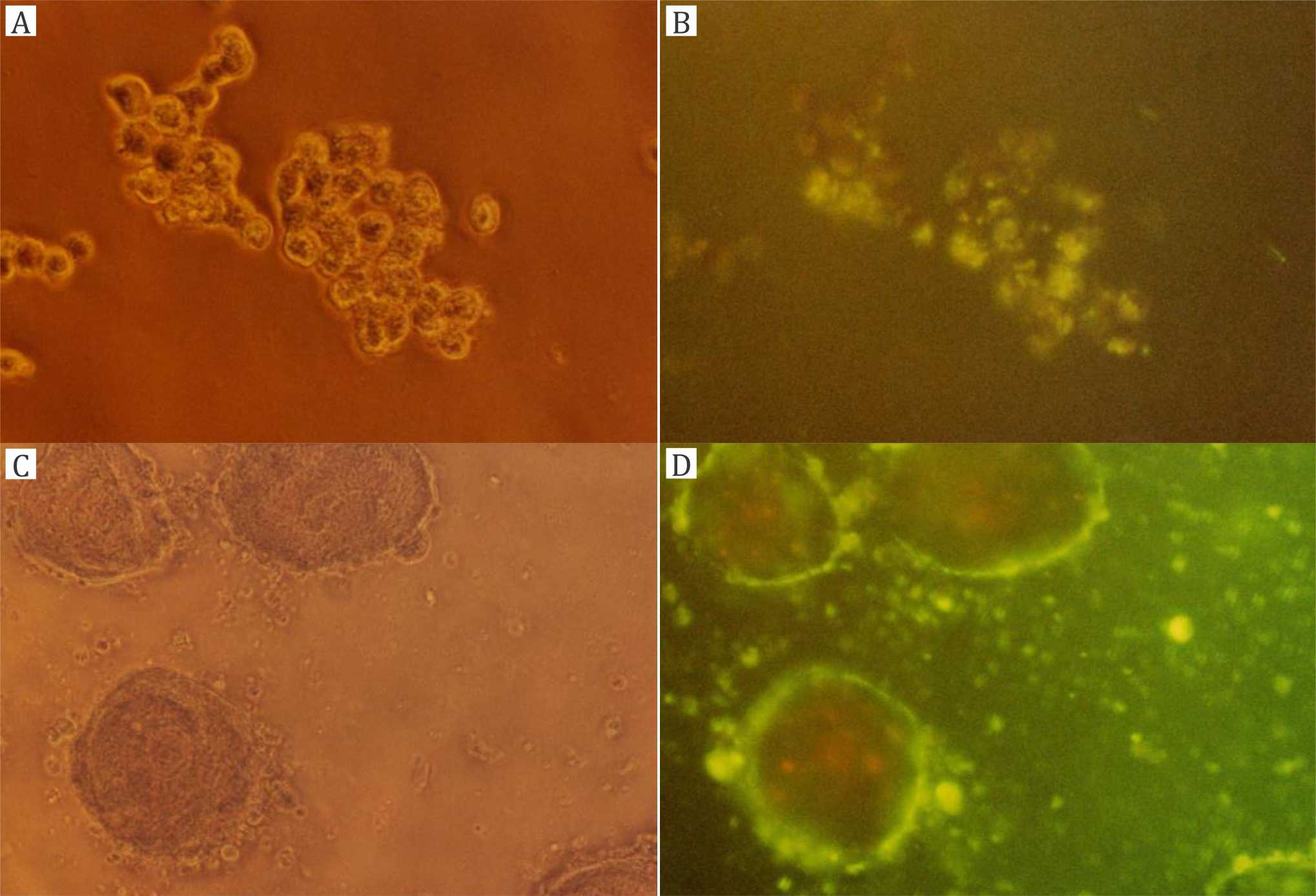
Figure 5. Experiments with T84 cell colonies: A, B) incubation with carboxyfluorescein (CF)-loaded dipalmitoylphosphatidylcholine (DPPC) liposomes for four hours (magnification 400x); C, D) incubation with CF-loaded tetraether lipid (TEL) liposomes for two hours (magnification 200x); A, C) phase contrast microscope; B, C) fluorescence microscope
Over a time course of up to four hours, cell-bound fluorescence did not strongly increase with DPPC (PCL) liposomes (Figures 3B, 4B, 5B). On the other hand, cell-bound fluorescence strongly increased from TEL liposomes within two hours (Figures 3D, 4D, 5D). The most relevant result is shown in figure 4D with the strongest cell-bound fluorescence in trypsinated cells after two hours of incubation with stable TEL liposomes. Hence, all other pictures should serve for comparison with the result in figures 4D and 5D as the confirmation of the result also in non-trypsinated adherent cell colonies.
DISCUSSION
Physicochemical characterization of TEL membranes and liposomes
This study describes the potential of tetraether lipid (TEL) extracted from the membranes of Thermoplasma species, laboratory stem DSM 1728 and cultured wildtype isolates from the Indonesian volcano Tangkuban Perahu. In archaeal membranes, TEL forms monolayers in similar thickness as bilayer membranes.7 This is a major reason for mechanical stability of TEL membranes under extreme environmental and experimental conditions18 since they do not break in the middle of the membrane as bilayers do.3,4 Mechanical and physicochemical stability are important for the administration of liposomes via GI or UG routes. The phytanyl chains of TEL are fully saturated and contain methyl side groups, which provide membrane fluidity on one hand.3,4 On the other hand, they are (different from unsaturated fatty acids) highly resistant towards oxidation and peroxidation.14 Membrane fluidity is regulated through these methyl side groups and pentacyclization within the phytanyl chains, which vary depending on growth temperature and external pH.7,19 Resistance of these membranes to acidic pH and their impermeability for protons (i.e., extremely low leakiness from TEL liposomes) are important for the stability of TEL liposomes in the acidic environment of the gastric fluid or urine.
Cytotoxicity, mutagenicity, toxicity and biodistribution in mice
The influence of liposomes from highly purified TEL (MPL) on a wide range of cell cultures was investigated in cytotoxicity screening including tests on mutagenicity and antimutagenic efficacy. None of the testing showed cytotoxic or mutagenic properties of TEL (MPL) or TEL liposomes.20 No toxicity was detected in mice in central nervous screening and the survival rates. The MPL-fed mice lived even longer than the controls.21 Biological stability of TEL-containing liposomes and biodegradation was investigated in the liver of mice.22 Although biotransformation of TEL needs further clarification, it is concluded that TEL liposomes can be safely applied also to humans at amounts needed for therapeutic purposes.
Release of 6-carboxyfluorescein (CF) and lipophilic dyes
Lipophilic dyes (Nile red and MBB) used in this study are transferred via intermembrane exchange from the liposomal membrane into the cell membrane. Membrane fusion and the uptake of liposomes into cells could not be proven in our experiments.
If the liposomes touch the cell surface or get attached to it, lipophilic dyes follow the pattern of ‘intermembrane exchange’ and in case of water-soluble CF, uptake into the cells occurs via ‘contact release’ from the liposomes at the cell surface. Our preliminary experiments with thiol-reactive MBB showed that it was readily transferred from liposomal TEL membranes into isolated erythrocyte membranes. The latter have thiol groups whereas liposomal TEL membranes do not. Hence, MBB exerted very low fluorescence emission in liposomal membranes. As soon as erythrocyte ghosts are present, MBB penetrates into the red cell membrane, reacts with thiols, and its fluorescence emission increases strongly.
The application of TEL to form more stable liposomes than common DPPC (PCL) appears advantageous, because the former are less leaky than the latter, especially at low pH (Table 1). Hence, the non-specific loss of CF from unstable and leaky DPPC (PCL) liposomes leads to diffuse fluorescence whereas the more cell-specific contact release from TEL liposomes leads to more intensive cell-bound fluorescence.
Biomedical application of archaeosomes and TEL liposomes
The liposomes used in this study were around 150 nm in diameter. Liposome technology with archaeal lipids does not differ in principle from the technology with common lipids. Archaeal TEL can even be used to stabilize liposomes of common lipids.22 The hydrolytic stability of TEL (MPL) liposomes and archaeosomes towards acidity and the low permeability of their membranes for protons3,4 provide wide possibilities for gastro-intestinal applications, in the first place for oral vaccination, especially since bacterial and archaeal membrane lipids seem to exert adjuvant properties in the GI mucosa.23,24 Further applications were shown by González-Paredes et al25 suggesting ‘archaeosomal hydrogels’ as novel delivery system for antioxidants.
After the intensive interaction of TEL liposomes with cultured T84 colon carcinoma cells seen in our experiments, we can add another possible application in the GI tract as antitumor delivery systems in the therapy of colon carcinomas. It is, of course, a long way from our in vitro experiments to in vivo animal studies and eventually to clinical application. For toxicological reasons, TEL liposomes and archaeosomes from archaeal membrane fractions must be purified from pigments and lipopolysaccharide complexes and from any other possibly toxic components. Lipid purification, in general, is very time-consuming and cost-intensive.6 Hence, for pharmaceutical application in oral drug delivery or oral vaccination, the use of sufficiently purified membrane fractions may be advantageous over highly purified single lipids. Purified membrane fractions both from Thermoplasma and Sulfolobus species form stable archaeosomes.26 Another possibility to reduce costs is the stabilization of (generally less expensive but instable) liposomes made of common bilayer-forming phospholipids with TEL at 2.5 mol% concentrations.22
Photodynamic therapy (PDT) using 5-ALA and its derivatives is based on heme biosynthesis and has been approved for treatment of various cancers. Treatment of cells with 5-ALA induces high bioactivity of the heme pathway and accumulation of porphyrins, mainly protoporphyrin IX (PpIX). Ferrochelatase, which is known to be less active in cancer cells, incorporates Fe2+ into PpIX and converts it into the photochemically inactive heme. Hence, PpIX mainly accumulates in cancer cells treated with 5-ALA. Subsequent illumination of these tumor cells at wavelength of 454 to 545 nm induces fluorescence of PpIX and thus differentiation from non-cancerous tissues. Since the fluorescent dye is also a photosensitizer, illumination will also induce the formation of singlet oxygen and other radicals which kill the respective tumor cells, but are less harmful to the surrounding healthy tissue.12 The technique, which is already successfully used for tumor diagnostics has already been approved for photodynamic cancer therapy.
Whereas the endogenous metabolic pathways support PDD and PDT, the way of administration of 5-ALA to the target tissue needs improvement and our idea comes in with liposomal systems for the delivery of fluorescent or photosensitizing dyes for diagnosis and treatment of colorectal cancers und urocyst transition cell carcinomas. Liposomal application in the UG tract will provide solutions aiming to intravesical administration of acid-stable archaeosomes via trans-urethral cystoscopic equipment. In the GI tract, the oral administration of acid-stable archaeosomes is particularly suitable to topical tumor treatment.
In conclusion, although stability of liposomes from archaeal TEL is much higher and leakiness much lower than that of liposomes from common phospholipids, the transfer of fluorescent dyes is more effective from these than from common DPPC liposomes, both for lipophilic dyes (Nile red, MBB) and hydrophilic CF. One reason may be that common liposomes already release part of their load unspecifically whereas stable liposomes from archaeal lipids keep their load and release it more specifically when they get in contact with target cells. A weakness of our study is that our fluorescence measurements could not be standardized for quantification. This should be the aim of following studies.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
This study was financially supported by the Deutsche Forschungsgemeinschaft in Germany and by URGE in Indonesia.
REFERENCES
- Handayani S, Santoso I, Freisleben HJ, Huber H, Andi, Ardiansyah F, et al. Archaeal life on Tangkuban Perahu - sampling and culture growth in Indonesian laboratories. Hayati J Biosci. 2012;19(3):150–4.
- Malik A, Santoso I, Yehuda A, Freisleben SKU, Wanandi SI, Huber H, et al. Characterization of Thermoplasma species cultured from sampling on Tangkuban Perahu, Indonesia. Microbiol Indones. 2014;8(1):16–23.
- Freisleben HJ. Tetraether lipid liposomes. In: Zimmer G, editor. Membrane structure in disease and drug therapy. New York: M. Dekker; 2000. p. 127–52.
- Freisleben HJ. Archaeosomes and tetraether lipid liposomes. Pharm Sci Res. 2012;9(1):53–65.
- Freisleben HJ, Henkel L, Gutermann R, Rudolph P, John G, Sternberg B, et al. Fermentor cultivation of Thermoplasma acidophilum for the production of cell mass and of the main phospholipid fraction. Appl Microbiol Biotechnol. 1994;40:745–52.
- Antonopoulos E, Freisleben HJ, Krisnamurti DGB, Estuningtyas A, Mulyanto C, Ridwan R, et al. Fractionation and purification of membrane lipids from the archaeon Thermoplasma acidophilum DSM 1728/10217. Separ Purific Technol. 2013;110:119–26.
- Luthfa Z, Freisleben HJ, Saleh R. Temperature and pHdependent molecular dynamics of Thermoplasma acidophilum tetraether lipid membrane in a computer-simulated model. Int J Mat Engin Technol. 2014;13(2):161–85.
- Kondo Y, Murayama Y, Konishi H, Morimura R, Komatsu S, Shiozaki A, et al. Fluorescent detection of peritoneal metastasis in human colorectal cancer using 5-aminolevulinic acid. Int J Oncol. 2014;45(1):41–6.
- Al-Naieb Z. New advances in aminolevulinic acid in urology. Med Surg Urol. 2014;3:e110.
- Zaak D, Hungerhuber E, Schneede P, Stepp H, Frimberger D, Corvin S, et al. Role of 5-aminolevulinic acid in the detection of urothelial premalignant lesions. Cancer. 2002;95(6):1234–8.
- Fukuhara H, Inoue K, Kurabayashi A, Furihata M, Shuin T. Performance of 5-aminolevulinic-acid-based photodynamic diagnosis for radical prostatectomy. BMC Urol. 2015;15:78–83.
- Berg K. Photosensitizers in medicine [Internet]. Norway: The Norwegian Radium Hospital; 2009 [cited 2015 Nov 2]. Available from: http://photobiology.info/Berg.html
- New RRC. Liposomes - a practical Approach. United Kingdom: IRL Press; 1990. p. 33–105.
- Choquet CG, Patel GB, Sprott GD. Heat sterilization of archaeal liposomes. Can J Microbiol. 1996;42(2):183–6.
- Oertl A, Freisleben HJ. Intermembrane exchange from liposomes of archaebacterial tetraether lipid into ghosts. Biol Chem Hoppe-Seyler. 1993;374:145.
- Antonopoulos E, Oertl A, Henkel L, Freisleben HJ. Liposomes of the main phospholipid from Thermoplasma acidophilum. Fluorescence determinations of interactions with cell membranes. Freiburg, Germany:, Fourth Liposome Research Days Conference University of Freiburg, Aug. 30 – Sept. 2, Book of Abstracts; 1995. P. 69.
- Corbo C, Molinaro R, Taraballi F, Toledano Furman NE, Sherman MB, Parodi A, et al. Effects of the protein corona on liposome–liposome and liposome–cell interactions. Int J Nanomedicine. 2016;11:3049–63.
- Vidawati S, Sitterberg J, Bakowsky U, Rothe U. AFM and ellipsometric studies on LB films of natural asymmetric and symmetric bolaamphiphilic archaebacterial tetraether lipids on silicon wafers. Colloids Surf B Biointerfaces. 2010;78(2):303–9.
- Shimada H, Nemoto N, Shida Y, Oshima T, Yamagishi A. Effect of pH and temperature on the composition of polar lipids in Thermoplasma acidophilum HO-62. J Bacteriol. 2008;190(15):5404–11.
- Freisleben HJ, Neisser C, Hartmann M, Rudolph P, Geck P, Ring K, et al. Influence of the main phospholipid (MPL) from Thermoplasma acidophilum and of liposomes from MPL on living cells: cytotoxicity and mutagenicity. J Liposome Res. 1993;3(3):817–33.
- Freisleben HJ, Bormann J, Litzinger DC, Lehr F, Rudolph P, Schatton W, et al. Toxicity and biodistribution of liposomes of the main phospholipid from the archaebacterium Thermoplasma acidophilum in mice. J Liposome Res. 1995;5(1):215–23.
- Purwaningsih EH, Arozal W, Jusman SWA. Uji stabilitas fisik, kimia dan biologik terhadap formulasi terbaru liposom tetra eter lipid (EPC-TEL 2,5) sebagai pembawa obat (drug carrier). Makara Kesehatan. 2007;11(2):84–9. Indonesian.
- Krishnan L, Sprott GD. Archaeosome adjuvants: immunological capabilities and mechanism(s) of action. Vaccine. 2008;26(17):2043–55.
- Li Z, Zhang L, Sun W, Ding Q, Hou Y, Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine. 2011;29(32):5260–6.
- González-Paredes A, Clarés-Naveros B, Ruiz-Martínez MA, Durbán-Fornieles JJ, Ramos-Cormenzana A, Monteoliva-Sánchez M. Delivery systems for natural antioxidant compounds: Archaeosomes and archaeosomal hydrogels characterization and release study. Intern J Pharm. 2011;421(2):321–31.
- Brown DA, Venegas B, Cooke PH, English V, Chong PLG. Bipolar tetraether archaeosomes exhibit unusual stability against autoclaving as studied by dynamic light scattering and electron microscopy. Chem Phys Lipids 2009;159:95–103.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id