
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
MMP-9, brain edema, and length of hospital stay of patients with spontaneous supratentorial intracerebral hemorrhage after hematoma evacuation along with the administration of tigecycline
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v25i4.1520 Med J Indones. 2016;25:221–7
Received: August 09, 2016
Accepted: September 25, 2016
Author affiliation:
1 Department of Neurosurgery, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
2 Department of Physiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Department of Clinical Pathology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
4 Department of Anasthesiology and Intensive care, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
5 Department of Neurosurgery, Faculty of Medicine, Universitas Diponegoro, dr. Kariadi Hospital, Semarang, Indonesia
6 Department of Neurology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
7 Clinical Epidemiology and Evidence-Based Medicine, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
8 Department of Pediatric, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Corresponding author:
Mohamad Saekhu
E-mail: saekhu2010@hotmail.com
Background
The high plasma level of matrix metalloproteinses–9 (MMP-9) is believed to disrupt the bloodbrain barrier (BBB) and cause brain edema, as well as increase patient’s length of hospital stay (LOS). Tigecycline showed ability to reduce the MMP-9 level on study in animals. This study aimed to evaluate whether tigecycline can reduce the plasma levels of MMP-9; brain edema; and LOS of patients with supratentorial spontaneous intracerebral hemorrhage (SSICH).
Methods
A randomized clinical trial (RCT) was conducted on 72 SSICH patients who underwent hematoma evacuation in eleven hospitals in Jakarta; 100 mg tigecycline (n=35) or 2 g fosfomycine (n=37) administered intravenously before skin incision as an prophylactic antibiotics to avoid post-operative infections. Plasma levels of MMP-9 were measured in all subjects before and on the first and seventh day after the surgery. Reduction of brain edema was assessed by comparing the extent of brain edema on computed tomography scan (CT scan) before and CT scan after surgery. The length of stay (LOS) was recorded at the time of hospital discharge either survive or death. Data were analyzed using Mann-Whitney and Chi-Square test.
Results
There were non-significant statistical differences between two groups in the proportion of subjects with reduced MMP-9 levels on the first day (48% vs 50%; p=0.902; OR=1.1) and seventh day after the surgery (33% vs 48%; p=0.296; OR=1.9); proportion of the subjects with brain edema reduction (86% vs 80%, p=0.58); LOS (median 12 days vs 13 days, p=0.256; LOS ≥15 days 40% vs 27%; p=0.243; OR=1.81; NNT=8).
Conclusion
On SSICH patients who underwent hematoma evacuation, tigecycline did not either reduce MMP-9 levels and brain edema or shorthen LOS.
Keywords
LOS, MMP-9, SICH, Tigecycline
Early mortality following spontaneous intracerebral hemorrhage (SICH) was related to transtentorial herniation due to hematoma with or without perihematomal brain edema mass effects.1 Surgical evacuation of the hematoma intracerebral is a reasonable choice to reduce the hematoma volume and the mass effect, as well as potentially reduce the production of neurotoxic substances triggered by the hematoma degradation products.2 However, surgical procedure was accompanied by additional injury.3,4 Neurological outcome after SICH remains unsatisfactory.5 One of several markers of brain injury either related to ICH6 or brain surgery4 is matrix metalloproteinase-9 (MMP-9). High level of MMP-9 is believed to be a predictor of hematoma growth, extent of brain edema and neurological deterioration.6 In traumatic brain injury, high levels of MMP-9 was to be a predictor of length of stay (LOS) and death in intensive care unit (ICU).7 On the other hand, MMP-9 inhibition is neuroprotective,8,9 as well as tigecycline reduce the MMP-9 level in animal study.10 This study aimed to confirm the changes of plasma level of MMP-9, brain edema, and LOS of pateints with supratentorial SICH (SSICH) after hematoma evacuation along with administration of single dose 100 mg tigecycline.
METHODS
This article is the second part of a randomized clinical trial (RCT) to assess the effects of tigecycline on patients with SSICH.11 Seventy two patients at eleven hospitals in Jakarta - Indonesia who prepared for hematoma evacuation were randomized into either receiving 100 mg tigecycline or 2 g fosfomycine intravenously as prophylactic antibiotics. The protocol of the study has been approved by Health Research Ethics Committee, Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo Hospital (No.493/PT02.FK/ETIK/2012).
The diagnosis of SSICH was based on clinical examinations and confirmed by computed tomography (CT) scan imaging. The indication for hematoma evacuation by surgery were SSICH patients with clinical deterioration as measured by GCS score less than 14 and hematoma valume ≥30 mL. The volume of hematoma was calculated with 1/2 (AxBxC) formula, where A is the greatest diameter of hematoma on CT scan image (on centimeter); B is the vertical diameter (90° to A (on centimeter); C is the amaount of slices on CT scan image (on centimeter).12 The evacuation of hematoma performed with craniotomy or craniectomy. Sample size was calculated by following formula:13
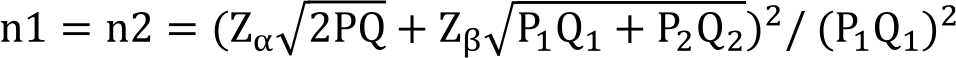
By taking P1=0.4 as the proportion of the effect of standar treatment, and P2=0.7 as proportion of the effect of tigecycline, α=0.05 and power 90%, a minimum os 22 subjects in each group were required.
Subject characteristics and outcomes were recorded as numeric and, or categorical data. The MMP-9 plasma level on postoperative day one and day seven changes the brain edema level on CT scan, and LOS at the time of hospital discharge were recorded as outcomes data. The MMP-9 plasma level was measured using the enzymelinked immunosorbent assay (ELISA), the reagent produced by Boster Biological in the United States. According to the previous study,14 normal value of MMP-9 was determined at 265 ng/mL.
The brain edema level was measured with blinded by main researcher or author (MS) based on perihematoma hypodensity thickness on the CT scan before surgery and hypodensity thickness at the former hematoma area in millimeter; hipodensity thickness ≤5 mm was set as mild degree of brain edema, 6 mm to 10 mm was set as moderate degree, and thickness more than 10 mm was set as severe degree. The difference of relative edema on CT scan before and after surgery was recorded as the changes of brain edema levels
Data from all of the subjects were included in the analysis. Numeric data was presented as mean value and data distribution while categorical data was presented as proportion (%). Hypothetical testing for numeric data was made using Mann–Whitney U test while categorical data were tested using Chi Square test. The statistical level of significance was set at p≤0.05. Relative clinical effectiveness was calculated using relative risk (RR), odds ratio (OR), and number needed to treat (NNT).
RESULTS
The number of samples collected during two years periode of study was 35 subjects in the treatment group (tigecycline) and 37 subjects in the control group (fosfomycine), the minimum number of sample required for this study exceeded.
Subject characteristics
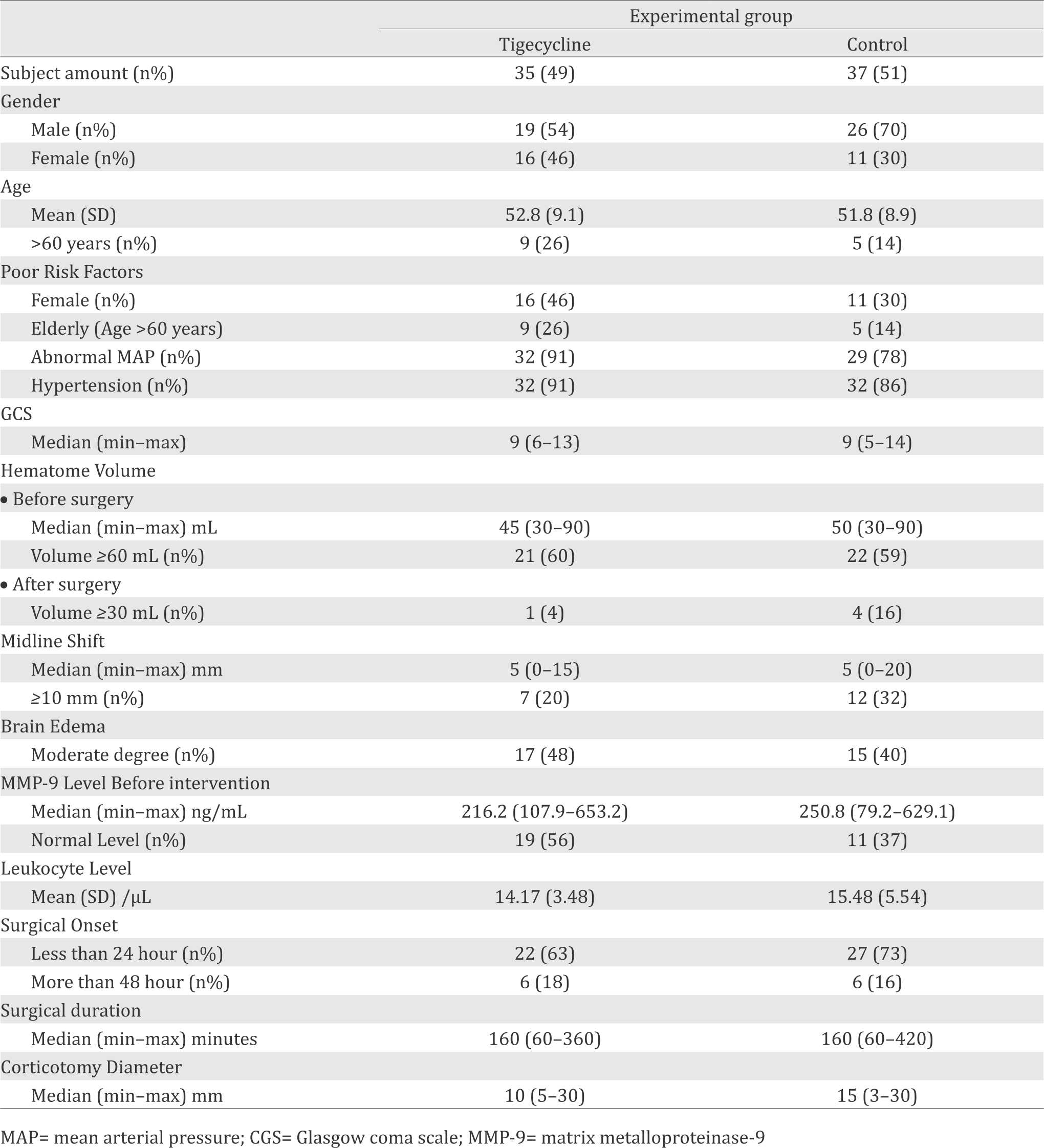
Age mean, Glasgow coma scale (GCS), and hematoma volume of subjects in both groups were equal. Risk factors for poor outcome such as elderly age (age over 60 years);14 female,15 history of hypertension and diabetes, and abnormal mean arterial pressure (MAP)17 were found greater in the tigecycline group. On the contrary, mid line shifting (MLS) of ≥10 mm, which is a sign of brain herniation and associated with poor outcome,18 was greater in the fosfomycine group.
Table 1. Subject characteristics
MMP-9 plasma levels
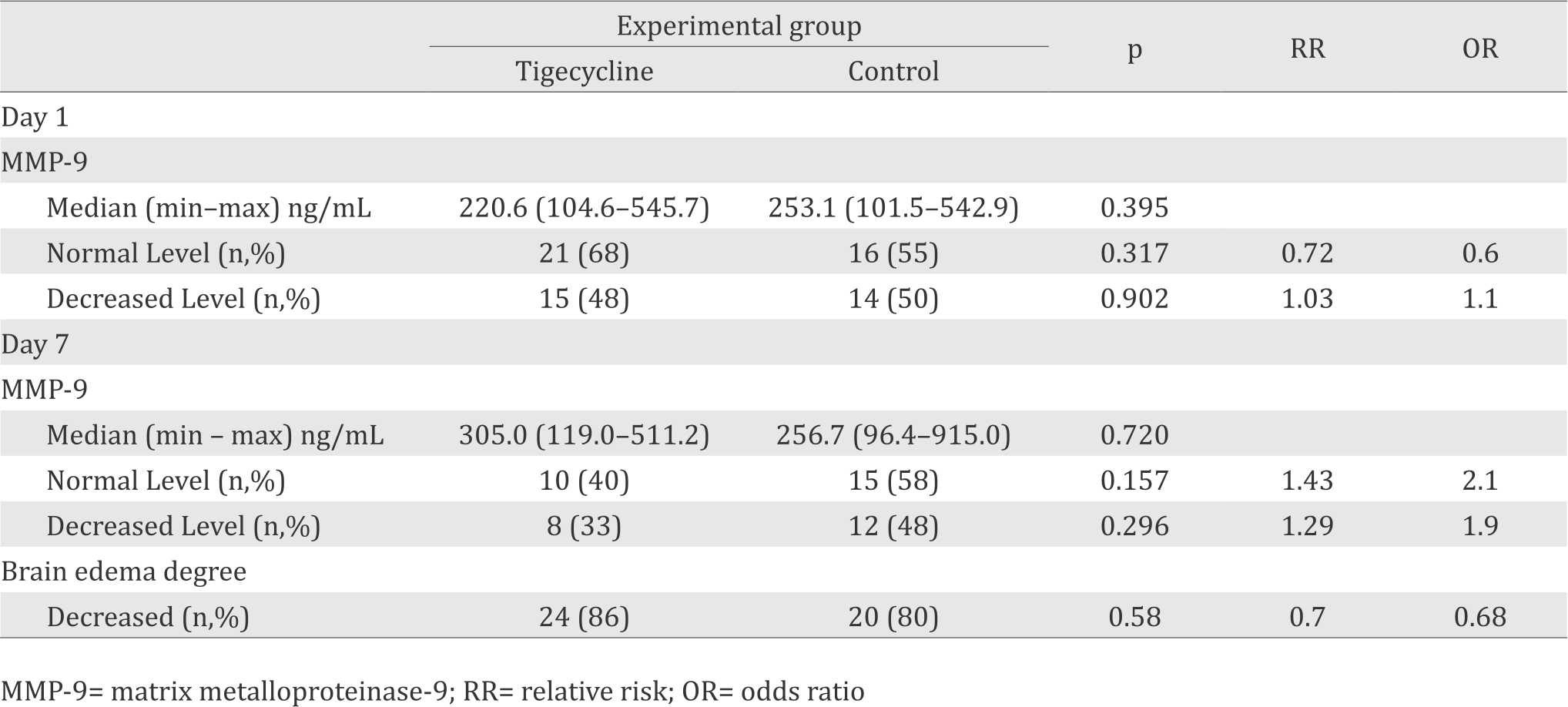
Changes of the MMP-9 plasma levels were analyzed based on mean value and proportion of subject with decreased level. Changes of plasma levels of MMP-9 are shown in table 2.
Table 2. Changes of plasma level of MMP-9 and brain edema
Post-operative day 1
Either the median of MMP-9 plasma level or proportion of subjects with decrease MMP-9 plasma level was statistically non significant different (table 2). Instead of lowering the plasma levels of MMP-9, tigecycline slightly increase the plasma MMP-9 levels. The chance for tigecycline to reduce the plasma levels of MMP-9 was 0.9 time compared to control group.
Post-operative day 7
Similar to the result on the day 1; on the day 7, either median MMP-9 plasma level or proportion of subjects with decrease MMP-9 plasma level were also statistically non significant. However, tigecycline group showed significant increase of the median MMP-9 plasma level; increased from 216.2 before surgery to 305.0 ng/mL on day 7 after surgery (increased 40%) compared to control group ie increased from 250.8 ng/mL before surgery to 256.7 ng/mL (increased 2%). The chances of patients in tigecycline group to decreased plasma levels of MMP-9 was 0.5 time compared to patients in the control group. Although statistically unsignificant, the increasing median level of plasma level of MMP-9, as well as a less proportion of patients experienced of reduce plasma level of MMP-9 in the tigecycline group may to be an early sign that tigecycline trigger an increase MMM-9 plasma level.
Brain edema
The CT scan on the day seven could be done in 74% subjects ie 89% subjects in tigecycline group and 65% subjects in control group. The mean reason for not to do CT scan were patients already death or unstable conditions. The chance of patients in tigecycline to reduce the brain edema levels is 1.5 times compared to patients in the control group.
Length of hospital stay (LOS)
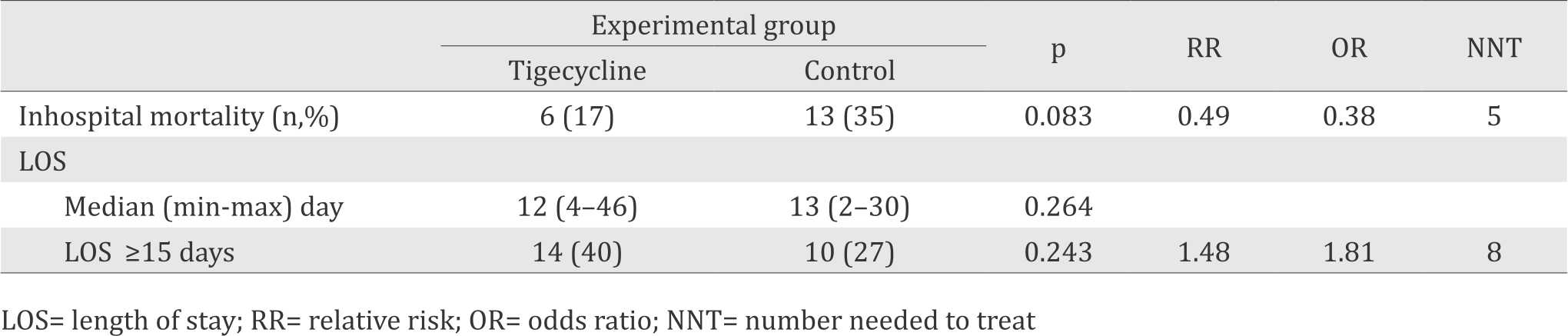
The chance of patients recieved tigecycline as prophyactic antibiotic experience LOS less than 15 days was 0.5 time compared to patients in the control group. However, control group showed more subjects experience an early death. Inhospital mortality before seven days after surgery in this study were 13 subjects; one subjects from tigecycline group and twelve subjects from control group (Table 3).
Table 3. Inhospital mortality and LOS
DISCUSSION
Phenolic β-diketone compound in the chemical structure of tetracycline has been known as metal chelator.19 Tetracycline derivates; doxycycline and minocycline has been known to have iron chelator activity.20 Matrix metalloproteinases are zincdependent endopeptidase, therefore, tetracycline and its derivative are believed to have an antimatrix metalloproteinases.21 Tigecycline is a new tetracycline derivate and its chemical structure also contains phenolic β-diketone.22 Study in animals showed that tigecycline reduced the MMP- 9 levels;10 however, result of the present study is not inline. Neither at the first nor at the seventh day after administration showed significant differences between the two groups. Inability of tigecycline to reduce the MMP-9 plasma levels in this study expected to be due to many factors such as different subjects, i.e. humans vs rats; different pathology, i.e. SICH vs staphylococcusinfected burn wound; terms or amount of tigecycline administered, i.e. single dose vs 21 times; MMP-9 measurement time, i.e. day one or day seven after single dose administration vs day one after 21 times or doses administration (at day twenty-second).9 MMP-9 also has twice-peak levels; the first peak is on the first three days after SICH attack and the second peak is on the seventh day.23 It is believed that the distinction between study in animals and study in humans stems from the aforementioned conditions.
MMP-9 is associated with disruption of BBB that promote brain edema.24 This study showed the changes of brain edema between tigecycline group and control group was not statistically significantly diffeerent. This result was in accordance with the non statistically significantly different in either median MMP-9 level or the proportion of subjects with decrease MMP-9 plasma level. However, there was slightly higher on the proportion of subjects with decrease brain edema (table 2). Considering that the proportion of subjects with decrease MMP-9 plasma level was lower in tigecycline group; as well as the median MMP-9 level was higher in tigecycline group; the comparability of severity of trauma related surgical procedure (surgical duration and the diameter of corticotomy; table 1); as well as comparability of hematoma volume before surgery, the amount of hematoma that evacuated was expected to be a main factor of the reduction of brain edema. The remaining hematoma volume after surgery ≥30 mL on CT scan control were 16% in control group compared to 4 % in tigecycline group. Therefore, the effects of tigecycline on reduction of brain edema in SSICH after surgical evacuation of hematoma could not be concluded.
Study on traumatic brain injury patients showed MMP-9 plasma level in the first 48 hours after onset was associated with endpoint intensive care unit (ICU) LOS.7 Meanwhile, in-hospital morbidity and complications were the main factors affecting the LOS of ICH patients.25,26 Considering that a longer LOS will increase the hospital cost, LOS could be an indicator of effectiveness and efficiency of a treatment choice as well as a hospital management.27 The present study showed the differences between the two groups; either the median of LOS or LOS more than 15 days, were statistically not significantly different. Previous study showed that early mortality was correlated with shorten LOS.28 This study showed 92% subjects who died prior to day seven after surgery was subjects from control group. It may be that the more higher proportion of early mortality in the control group was be a factor of more higher proportion of subjects in the tigecycline group that having LOS ≥15 days. Overall, this study could not conclude the effects of tigecycline on LOS of SSICH patients after surgical evacuation of hematoma, whether shorten or extend the LOS.
Contrary to what has been reported in animal study, instead of reducing the plasma levels of MMP-9, tigecycline increased MMP-9 plasma levels on day seven, with the median of 40% in tigecyline group, and 2 % on control group. The proportion of subjects with normal plasma level of MMP-9 on tigecycline group was decrease by 29%. In contrary, the control group showed increase proportion of subjects with normal level of MMP-9 by 57%. The proportion of subjects with increase plasma levels of MMP-9 in tigecycline group was higher than control group (67% vs 52% respectively). This trend was in accordance with study in human retinal pigment epithelial cells culture that showed minocycline; the prototype of tigecycline, promote the increase of MMP-9 levels.29 Beside having dual peak levels, MMP-9 also has dual roles. On the first three days or the first peaks, MMP-9 play a role in pathologic process, ie disruption of BBB. Meanwhile, on the second peak, MMP-9 plays role in neuronal recovery.23 The increase levels of MMP-9 is needed for healing process as well as neurogenesis by promoting migration of activated microglia30 and endogen stem cells.31,32 Endogenous neurogenesis was believe to be a powerful tool to repair the brain after SICH.33 However, this study could not conclude the effects of tigecycline on short-term clinical outcome, mainly on patient’s LOS.
There were some limitations in this study. This RCT involving 11 hospitas in Jakarta, This study showed the median of LOS was comparable with the studies in US (14.9 days)34 and Germany (15 days).26 The median of LOS in the present study was 12 days in tigecycline group and 13 days in control group. According to the formula for sample size, this study needs 22 subjects each group. During two years peiode of study, 72 subjects was colected. Compared to the study in US that involves 45,159 subjects and study in Germany that involves 1,405 subjects, subjects involved on this study was only slightly. However, to the best of our knowledge, this is the first RCT of effetcs of tigecycline on MMP-9 levels as well as LOS of SSICH patients that surgically treated in Indonesia. Considering that tigecycline clinically effective to reduce in-hospital mortality,11 further study is needed to determine the effects of multiple adminitrations of tigecycline on clinical outcomes in meddle to long-term clinical outcomes.
In conclusion, the administration of single dose of 100 mg tigecycline for SSICH patients that surgically treated to evacuate the hematoma has not been able to significantly change MMP-9 plasma levels, brain edema, and the LOS. However, the increase of MMP-9 plasma levels on the day seven in tigecycline group need further studies to explore the effects of tigecycline for better recovery after SICH.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The author would like to thank Prof. Sarwono Waspadji, M.D., Ph.D. and all medical staff members of the Department of Neurosurgery, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, as well as all hospitals that have participated in this study.
REFERENCES
- Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15(3):492–6.
- Minematsu K. Evacuation of intracerebral hematoma is likely to be beneficial. Stroke. 2003;34(6):1567–8.
- Sulejczak D, Grieb P, Walski M, Frontczak-Beniewicz M. Apoptotic death of cortical neurons following surgical brain injury. Folia Neuropathol. 2008;46(3):213–9.
- Frontzack-Beniewicz M, Chrapusta SJ, Sulejzak D. Long-term consequences of surgical brain injury characteristics of the neurovascular unit and formation and demise of glial scar in a rat model. Folia Neuropathol. 2011;49(3):204–18.
- Meyer S, Verheyden G, Brinkmann N, Dejaeger E, de Weerdt W, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: Followup of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. 2015;46:1613–9.
- Petrovska-Cvetkovska D, Dolnenc-Baneva N, Nikodijevic D, Chepreganova-Changovska T. Correllative study between serum matrix metalloproteinase – 9 value and neurologic deficit in acute primary supratentorial intracerebral haemorrhage. Sec Med Sci. 2014.
- Copin JC, Rebetez MML, Turck N, Robin X, Sanchez JC, Schaller K, et al. Matrix metalloproteinae 9 and cellular fibronectin plasma concentrations are predictors of the composite endpoint of length of stay and death in the intensive care unit after severe traumatic brain injury. Scan J Trauma, Resc and Emerg medicine.2012;80(83).
- Yong VW, Giuliani F, Xue M, Bar-Or A, Metz LM. Experimental models of neuroprotection relevant to multiple sclerosis. Neurology. 2007;68(Suppl 3):S32–7.
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128(Pt7):1622–33.
- Simonetti O, Cirioni O, Lucarini G, Orlando F, Ghiselli R, Silvestri C, et al. Tigecycline accelerates staphylococcal-infected burn wound healing through matrix metalloproteinase-9 modulation. J Antimicrob Chemother.2012;67:191–201.
- Saekhu M, Mahyuddin H, Ronokusumo TAS, Sastroasmoro S. Tigecycline reduces tumor necrosis factor alpha level and inhospital mortality of patients with spontaneous supratentorial intracerebral hemorrhage. Med J Indones. 2016;25(2):69–75.
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–5.
- Madiyono B, Moeslichan S, Sastroasmoro S, Budiman I, Purwanto SH. Perkiraan besar sampel. In: Sastroasmoro S, Ismael S, editors. Dasar-dasar metodologi penelitian klinis. 5th ed. Jakarta: CV Sagung Seto; 2014. p. 352–95.
- Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessa I, Goussetis E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinaseassociated lipocalin (NGAL) and their complex MMP-9/ NGAL in breast cancer disease. BMC Cancer. 2009;9:390.
- Umeano O, Phillips-Bute B, Hailey CE, Sun W, Gray MC, Roulhac-Wilson B, et al. Gender and age interact to affect early outcome after intracerebral hemorrhage. PLoS One. 2013;8(11):e81664.
- Ganti L, Jain A, Yerragondu N, Jain M, Bellolio MF, Gilmore RM, et al. Female gender remains an independent risk factor for poor outcome after acute nontraumatic intracerebral hemorrhage. Neurol Res Intl. 2013;2013(219097):1–7.
- Tetri S, Juvela S, Saloheimo P, Pyhtinen J, Hillbom M. Hipertension and diabetes as predictor of early death after spontaneous intracerebral hemorrhage. J Neurosurg. 2009;110(3):411–7.
- Togha M, Bakhtavar K. Factor associated with inhospital mortality following intracerebral hemorrhage: a three year study in Tehran, Iran. BMC Neurology. 2004;4(9):1–5.
- Sakaguchi T, Toma M, Yoshida T, Omura H, Takasu H. Metal shelate with tetracycline derivates part VII: The structure of tetracycline chelates. Chem Pharma Bulletin. 1958;6(1):1–9.
- Grenier D, Huot MP, Mayrand D. Iron-chelating activity of tetracycline and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother. 2000;44(3):763–6.
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotics properties and their clinical implication. J Am Acad Dermatol. 2006;54(2):256–65.
- da Silva LM, Nunes Salgado HR. Tigecycline: a review of properties, applications, and analitycal methods. Ther Drug Monit. 2010;32(3):282–8.
- Chang JJ, Emanuel BA, Mack WJ, Tsivgoulis G, Alexandrov AV. Matrix metalloproteinase-9: dual role and temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23(10):2498–505.
- Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute eschemic stroke. Front Neurol. 2013;4(32):1–15.
- Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-hospital medical complication, length of stay, and mortality among stroke unit patients. Stroke. 2011;42(11):3214–8.
- Stein M, Misselwitz B, Hamann GF, Kolodziej MA, Reinges MHT, Uhl E. Defining prolonged length of acute care stay for surgically and conservatively treated patients with spontaneous intracerebral hemorrhage: a populationbased analysis. BioMed Res Int. 2016;2016(9095263):1–6.
- Chan CL, Ting HW, Huang HT. The definition of a prolonged intensive care unit stay for spontaneous intracerebral hemorrhage patients: an application with National Health Insurance Reasearch database. BioMed Res Int. 2014;2014(891725):1–9.
- Kim HT, Lee JM, Koh EJ, Choi HY. Surgery versus conservative treatment for supratentorial intracerebral hemorrhage in spot sign positive patients. J Korean Neurosurg Soc. 2015;58(4):309–15.
- Hollborn M, Wiedemann P, Bringmann A, Kohen L. Chemotactic and cytotoxic effects of minocycline on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(5):2721–9.
- Lively S, Schlichter LC. The microglial activation state regulates migration and role of matrix-dissolving enzymes for invasion. J Neuroinflammation. 2013;10:75.
- Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote differentiation and migration of adult neural progenitor in response to the chemokines. Stem Cells. 2008;26(12):3139–49.
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, et al. Involment of matrix metalloproteinase in neuroblast migration from the subventricular zone after stroke. J Neurosci. 2006;26913):3491–5.
- Andres RH, Guzman R, Ducray AD, Mordasini P, Gera A, Barth A, et al. Cell replacement therapy for intracerebral hemorrhage. Neurosurg Focus. 2008;24(3–4):E15.
- Patil CG, Alexander AL, Hayden Gephart MG, Lad SP, Arrigo RT, Boakye M. A population-based study of inpatient outcomes after operative managemant of nontraumatic intracerebral hemorrhage in the United States. World Neurosurg. 2012;78(6):640–5.
Copyright @ 2016 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id