
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
High frequency of NAT2 slow acetylator alleles in the Malay population of Indonesia: an awareness to the anti-tuberculosis drug induced liver injury and cancer
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i1.1563 Med J Indones. 2017;26:7–13
Received: September 13, 2016
Accepted: April 04, 2017
Author affiliation:
1 Department of Histology, Faculty of Medicine, YARSI University, Jakarta, Indonesia
2 Genomic Medicine Research Group, YARSI Research Institute, YARSI University, Jakarta, Indonesia
3 Department of Patology Anatomy, Faculty of Medicine, YARSI University, Jakarta, Indonesia
4 Department of Pharmacology, Faculty of Medicine, YARSI University, Jakarta, Indonesia
5 The Indonesian Pharmacogenomics Working Group
Corresponding author:
Rika Yuliwulandari
E-mail: rika.yuliwulandari@yarsi.ac.id
Background
Arylamine N-acetyltransferase 2 (NAT2) polymorphism was previously reported to have association with the risk of drug toxicities and the development of various diseases. Previous research on the Indonesian population, especially Javanese and Sundanese, showed that there were 33% NAT2 slow acetylator phenotype. The aim of this study was to map the NAT2 variation in the Malay ethnic to gain a deeper insight into NAT2 haplotypic composition in this ethnic.
Methods
50 healthy samples from the Indonesian Malay ethnic were obtained. They were interviewed about their ethnic backgrounds for the last three generations. DNA was extracted from peripheral blood and NAT2 genotyping was done using the PCR direct Sequencing. Data were compiled according to the genotype and allele frequencies estimated from the observed numbers of each specific allele. Haplotype reconstruction was performed using PHASE v2.1.1 software.
Results
We found 7 haplotypes consisting of 6 SNPs and 14 NAT2 genotype variations in Indonesian Malay population. The most frequent allele was NAT2*6A (38%) which was classified as a slow acetylator allele. According to bimodal distribution, the predicted phenotype of the Malay population was composed of 62% rapid acetylator and 38% slow acetylator. According to trimodal distribution, the predicted phenotypes for rapid, intermediate and slow acetylators were 10%, 52% and 38% respectively.
Conclusion
Our result indicates the presence of the allelic distribution and revealed the most frequent acetylator status and phenotype for the Indonesian Malay population. The result of this study will be helpful for future epidemiological or clinical studies and for understanding the genetic basis of acetylation polymorphism in Indonesia.
Keywords
Indonesian Malay ethnic, NAT2, polymorphism, slow acetylator
The arylamine N-acetyltransferase 2 (NAT2) gene, located on chromosome 8p22, is autosomal dominant and intronless, with a single open reading frame of 870 bp.1 The genetic variant in NAT2 gene may vary between ethnicities and influence individual variation in cancer susceptibility, individual response to environmental toxins and the effectiveness of medication treatment.2 Previous reported studies showed that NAT2 slow acetylator phenotypes are associated with disease risks and drug toxicity.3 The NAT2 slow acetylator phenotypes have been investigated to have association with cancer risk4 and isoniazid-induced hepatotoxicity in tuberculosis treatment.5 According to Sabbagh et al6 the slow acetylator status is more frequent in all populations in Europe in which more than 50% of individuals (59% on average) carry the slow acetylator genotype. High prevalence of slow acetylator phenotype is also observed in many parts of Asia (Middle East, India, North Asia, and Southeast Asia). In contrast, this phenotype is much rare in Northeast Asia (18% in average) owing to the high prevalence of the fast NAT2*4 haplotype in this group of populations. The prevalence of slow acetylators is highly heterogeneous in Africa and in America, with striking differences among populations, even at a small geographic scale.6
A study of NAT2 in Indonesia has been conducted in Javanese and Sundanese population.1 It revealed that slow acetylator variants were frequently observed in those populations. However, Indonesia consists of more than 300 ethnic groups with major ethnics are Javanese (40%), Sundanese (15.50%), Malay (3.7%), Batak (3.58%), Madurese (3%), and Betawi (2,88%).7 Considering the wide ethnic diversity in Indonesia, a study on the NAT2 gene is important to make the diverse ethnic groups in the Indonesia population more aware of their genetic risk. Therefore, this study was designed to explore NAT2 polymorphism in other ethnics, especially the Indonesian Malay ethnic as the third largest ethnic in Indonesia.
METHODS
Sample collection
We collected samples from 50 medical students of Universitas YARSI by random sampling in the Faculty of Medicine, Universitas YARSI. A peripheral blood (5 mL) from healthy participants was collected into ethylenediaminetetraacetic acid (EDTA) tubes and stored in a minus 20°C freezer until further processing. All the participants were interviewed for their ethnic backgrounds and only subjects of Indonesian Malay origin for three past generations were recruited for the study. They comprised 13 male and 37 female subjects with age ranges between 17 and 21 years. A written informed consent in the Indonesian language was obtained from each participant in this study. The protocol of this study has been approved by the research ethics committees of YARSI University, Jakarta, Indonesia (No. 001/ETIK/BIA/VI/2006).
NAT2 genotyping
All of the processes for genotyping of NAT2 gene were done in YARSI Research Center, YARSI University, Jakarta, Indonesia. Genomic deoxyribonucleic acid (DNA) extraction was done using QIAamp DNA blood mini kits (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genotyping was done using the polymerase chain reaction (PCR) direct sequencing method. PCR was performed on a Sensoquest Labcycler Thermocycler (Sensoquest, Gottingen, Germany) and composed of 25-μl reaction mixture containing 20 ng of genomic DNA, 1× of FastStart 10× PCR Buffer with 20 mM MgCl2 (Roche Applied Science, Penzberg, Germany), 200 μM of dNTPs, 0.2 μM of each primers (Forward and Reverse) and 1 U of FastStart Taq DNA polymerase (Roche Diagnostics, Mannheim, Germany). PCR profiles were as follows: initial denaturation at 96°C for four minutes; 40 cycles of denaturation at 96°C for 30 s, annealing at 55°C for 30 seconds and elongation at 72°C for 30 seconds; and a final elongation step at 72°C for five minutes. Forward and reverse primers used for PCR and DNA direct sequencing were based on a previously published report.1 Direct DNA sequencing was performed using 310 genetic analyzer (Applied Biosystems, California, United States of America).
Statistical analysis
Data were compiled according to the genotype and allele frequencies estimated from the observed numbers of each specific allele. The statistical analyses of the allelic and genotypic frequencies were conducted using a Chi-squared test. Haplotype reconstruction was performed using PHASE v2.1.1 software that implements a Bayesian statistical method.8 NAT2 genotypes and predicted acetylator phenotypes were inferred from the reconstructed haplotype by comparing to the human NAT2 allele database at http://nat.mbg.duth.gr/HumanNAT2alleles_2013.htm. We grouped the predicted phenotype into bimodal and trimodal distribution groups. The bimodal distribution consists of rapid acetylator (RA) and slow acetylator (SA), while the trimodal distribution comprises RA, intermediate acetylators (IA) and SA. We considered RA as homozygous for rapid allele, IA as heterozygous between rapid allele and slow allele, and SA as homozygous for slow allele.
RESULTS
In total, seven different allelic variants of the NAT2 gene were identified in the Indonesian Malay ethnic population based on the NAT2 haplotype construction (Table 1). NAT2*6A allele (38%) is the most frequent allele in this population. According to the human NAT2 database (http://nat.mbg.duth.gr/HumanNAT2alleles_2013.htm), NAT2*4, NAT2*12A and NAT2*13 are classified as rapid-acetylator alleles, whereas NAT2*5B, NAT2*6A, NAT2*6B, and NAT2*7B are classified as slow-acetylator alleles Table 1.
Table 1. Allele frequency of NAT2 healthy in Indonesian Malay ethnic population
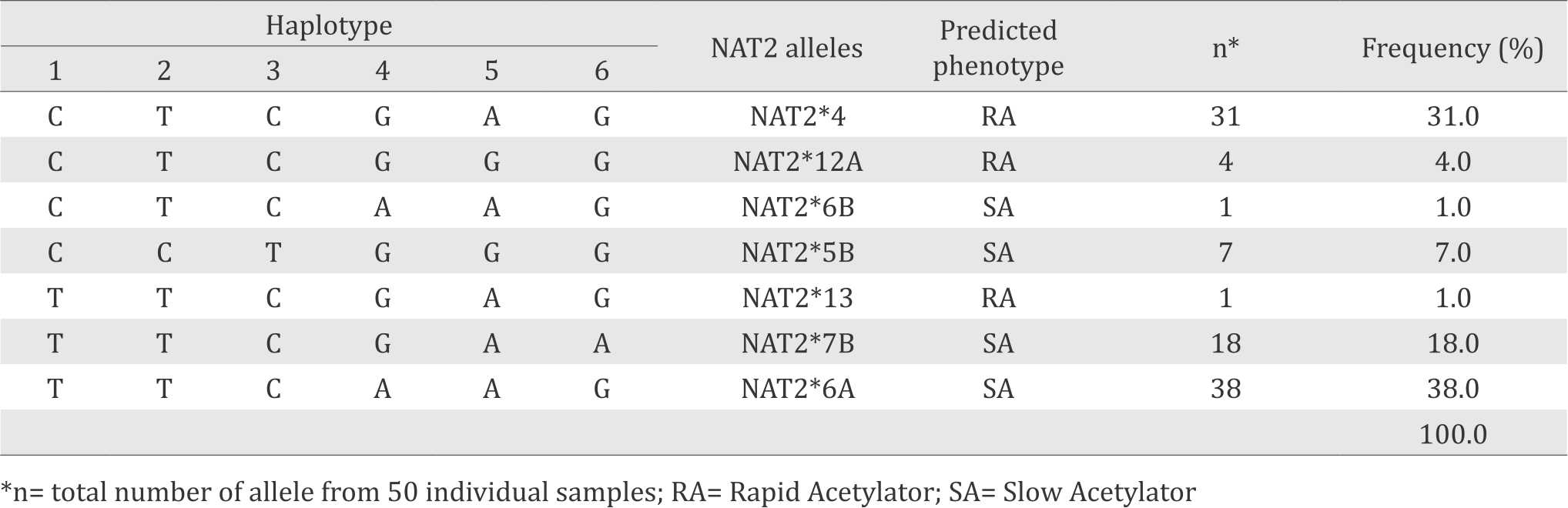
In this study we also found that in total there are 14 genotype variants of NAT2 gene. Most of them were predicted as slow acetylator phenotypes. Predicted phenotype showed that 38% of the studied groups were SA and 62% were RA according to bimodal distribution. According to trimodal distribution, the frequencies of predicted phenotype were 10%, 52%, and 38% for rapid, intermediate, and slow acetylators, respectively. The genotype variants and their corresponding predicted phenotype profiles were presented in Table 2.
Table 2. Frequency of NAT2 genotypes and predicted phenotypes in Malay ethnic
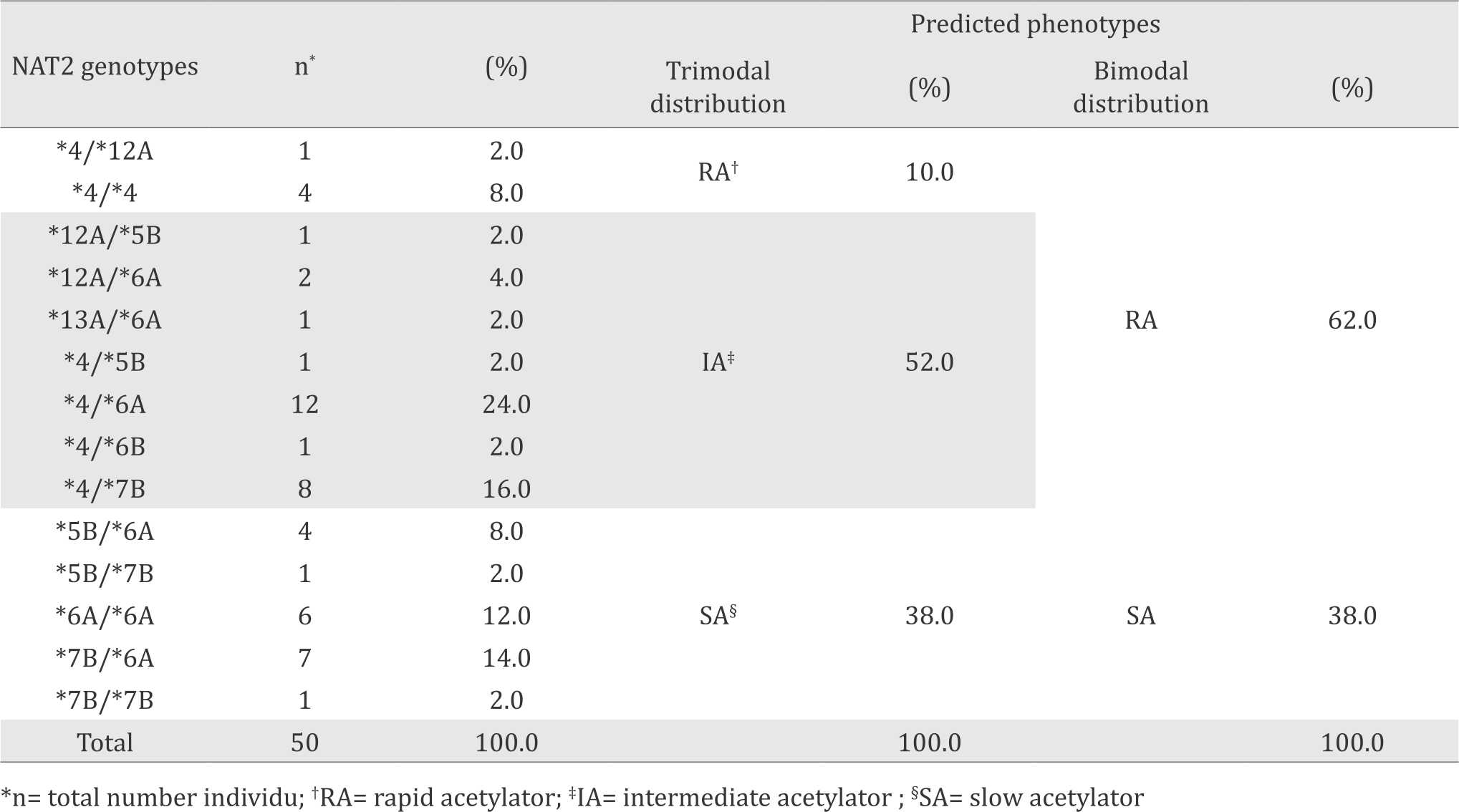
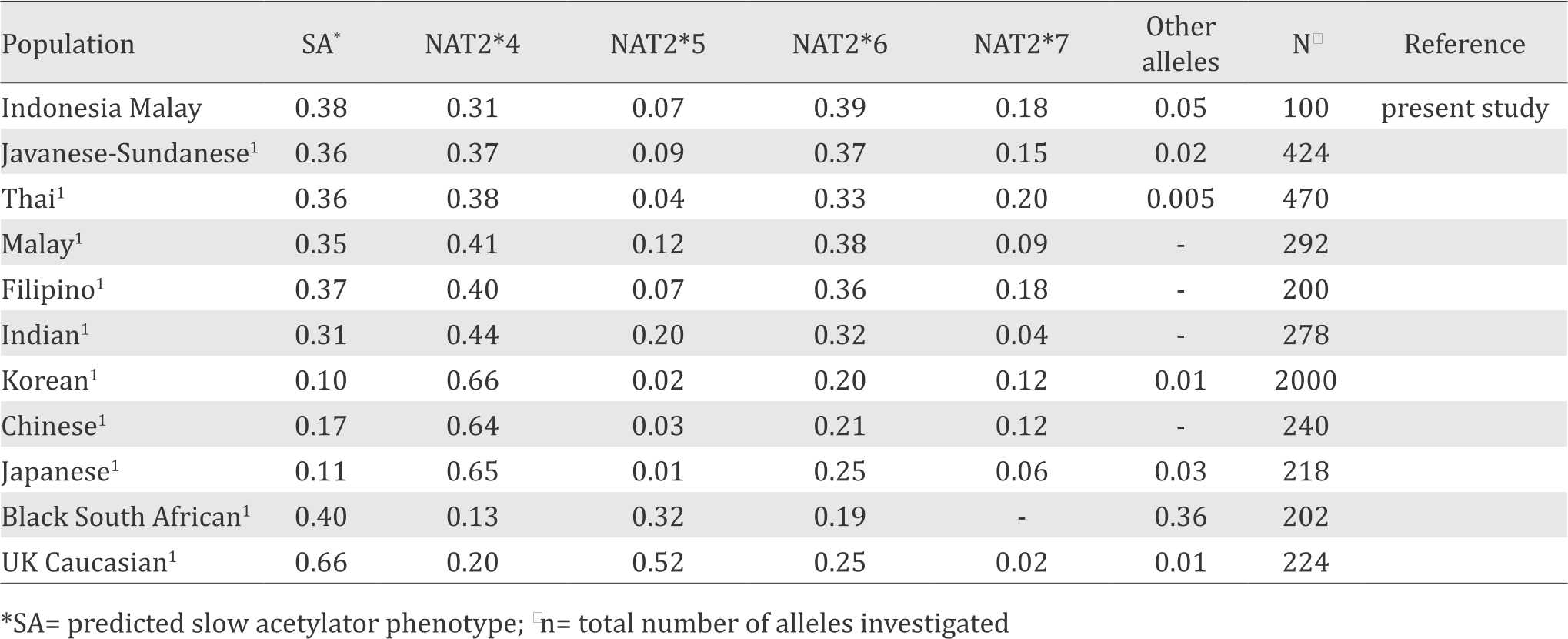
Table 3 showed the comparison of NAT2 allele frequencies of the Indonesian Malay population and other populations. The frequency of slow acetylators in the Indonesian Malay population was relatively similar to previous data in the Indonesian Javanese-Sundanese populations and other Southeast Asian populations. The frequencies of slow acetylators in Indonesian and other Southeast Asian populations were higher than those in Northeast Asian populations but lower than those in Caucasians and Africans Table 3.
Table 3. Distribution of NAT2 alleles in various human population
DISCUSSION
Haplotypes have a more important role than individual single nucleotide polymorphisms (SNPs) in genes that contain various SNPs in high linkage disequilibrium (LD), such as NAT2 gene. In this kind of gene, the haplotype structure is the principal determinant of phenotypic consequences.9 In NAT2 gene, generally there are seven single SNPs that affect the metabolic capacity of this enzyme. The combination of these SNPs, called by haplotypes (or NAT2 alleles) can be assigned to predict acetylation phenotypes either in bimodal distribution or trimodal distribution.10,11 In this study, we found in total seven different haplotypes in the Indonesian Malay population (Table 1). NAT2*4 is a wild type allele and known as a RA allele, the same type acetylator with NAT2*12A and NAT2 13. Variant alleles such as NAT2*5A, NAT2*6A, NAT2*6B, and NAT2*7B are categorized as SA alleles.
Slow acetylator genotypes that we found in the present study were showed in Table 2. SA phenotypes were known to increase the risk of cancer and drug toxicity. A meta-analysis study showed that the SA genotype of NAT2 was significantly associated with increased risk of antituberculosis drug hepatotoxicity in East Asians, South Asians, Brazilians and Middle Eastern when stratified by ethnicity.12 Another meta-analysis study reported significant association between slow acetylator and prostate cancer in Asians.13
Many studies showed that NAT2 slow acetylator alleles are associated with several disease risks. A previous reported study revealed that NAT2*6A is an important genetic marker for the risk of hepatotoxicity due to anti-tuberculosis treatment in Chinese, Japanese, Korean12 and Indonesian Javanese and Sundanese.5 A meta-analysis of 26 case–control studies also reported that the NAT2 SA genotype was a risk factor of antituberculosis drug induced liver injury (AT-DILI), but the associations are diverse in different ethnic populations. Significant results were found in East Asians, South Asians, Brazilians and Middle Eastern, but not in Caucasians.12 It was expected that subjects with NAT2 SA may have a decreased activity of NAT2 which affects the acetylation of both isoniazid and hydrazine; therefore they are more susceptible to AT-DILI.
Another reported study found a significant association between NAT2*6A and age‐related hearing impairment (ARHI) in the Turkish population14 the general European population15 and among the Hispanic population.16 Unal et al14 revealed an increased risk to 15.2-fold for the development of ARHI in individuals with a NAT2*6A allele. NAT2*6A allele was predicted to slow down the detoxification mechanism and lead to an accumulation of xenobiotics in the inner ear that might increase the number of acquired mitochondrial mutation, consequently leading to cell damage and hearing loss.15
NAT2 slow acetylator alleles also have an impact to the risk of cancer. NAT2*6A and NAT2*7A/B increases the risk to prostate cancer in the Turkish population.17 Another reported study showed that the presence of NAT2*7 allele might be a potential risk factor for the development of brain tumors in Taiwan.18 A slow acetylation profile also increased risk of developing gastric cancer and bladder cancer in Brazil.4 Reported studies by Moore et al19 and Figueroa et al20 revealed the impact of NAT2 SA on bladder cancer risk in a person who was exposed to carcinogenic NAT2 substrates such as aromatic amines from tobacco smoke.19,20 Additionally, a meta-analysis study by Moore et al19 also reported that the NAT2 slow acetylation genotype was associated with bladder cancer risk (OR=51.31; 1.01–1.70). Increased risk of bladder cancer was greater among heavy smokers (OR=52.11; 1.33– 3.55) than light smokers (OR=50.96; 0.61–1.53).19
Interestingly, recent evidence suggests that the NAT2*6 haplotype cluster is related with the slowest acetylation capacity in vivo, and that the homozygous genotype NAT2*6/*6 thus defines a new category of ‘ultra-slow’ acetylators.11,21 In vivo studies with a widely used caffeinebased assay in Europeans found 35%21 and 46%,11 respectively, decreased NAT2 capacity in NAT2*6/*6 genotypes compared to another NAT2 slow genotypes *5/*5.22 Anti-tuberculosis druginduced hepatotoxicity risk has been shown to be particularly high in carriers of the NAT2*6/*6 genotype.23 Similarly, the ultra-slow genotype, and not the common slow NAT2 genotype, has been significantly associated with an increased risk of urinary bladder cancer.11 A recent study by Selinski et al24 also suggests an association of ultra-slow NAT2 with higher cognitive auditory functions in elderly persons.
The distribution of NAT2 alleles in various populations was showed in Table 3. The frequency of NAT2 slow acetylation in Malay population was similar to our previous studies with the Javanese and Sundanese population.1 The NAT2 SA alleles frequency in our present study also resembled other Southeast Asian populations (Table 3). Table 3 also showed that the frequency of NAT2 SA alleles in Northeast Asian population are the lowest compared to Southeast Asian, Caucasian and African populations. Further genetic characterization of different populations and development of preventive strategies adopted for ethnicities with different genetic backgrounds are needed to deal with the emerging health care problems in developing multiethnic societies.
The high frequency of NAT2 slow acetylator in the Indonesian Malay population and in Javanese and Sundanese populations shown by previous research1 should be our concern to prevent the risk of cancer and drugs toxicity, especially hydrazine-based drugs which are metabolized by NAT2. Isoniazid (INH) is one example of hydrazine-based drugs and is used mostly to tuberculosis (TB) patients, since INH is one of the drugs that is used in standard regiment for TB treatment. Recent reports have shown that NAT2 SA leads to the development of hepatotoxicity in patients receiving tuberculosis drugs in Indonesia, especially in the Javanese and Sundanese population.5 Similar finding also showed in East Asians, South Asians, Brazilians and Middle Eastern.12 This is because SA types break down the drugs at a very slow rate while fast acetylator types work much more quickly. Consequently SA patients are at risk of toxic effect because more drugs reaches their plasma than those with fast acetylators. On the other hand, fast acetylator patients are at risk of having too little drug in their plasma, which reduces the effectiveness of the drugs.25
In conclusion, our study showed the distribution of NAT2 genetic polymorphism in the Malay population of Indonesia. Additionally, this study provided a background for further epidemiological studies to evaluate the impact of NAT2 genotype/ phenotype polymorphism to several disease risks. This study will also be helpful for understanding the genetic basis of acetylation polymorphisms in Indonesian. However, this study needs to be replicated with a larger sample size that includes the Indonesian Malay ethnic from their original place so that we can describe the genetic profile of the Indonesian Malay ethnic. Such a study may also find a rare and specific allele of NAT2 in the Indonesian Malay Ethnic. Further research has to be conducted with another major ethnic in Indonesia for understanding the genetic basis of NAT2 polymorphism in Indonesians. The genetic basis of NAT2 polymorphism in Indonesia can lead to the development of diagnostic kit that allows clinicians to predict patients’ risk to adverse effect of arylamine- and hydrazine-based drugs which are metabolized by NAT2.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
This project was funded by a grant from the Indonesian Directorate General of Higher Education (DIKTI) of the Ministry of Higher Education, Research and Technology of the Republic of Indonesia. We are thankful to the YARSI foundation for their support, the YARSI Genomic Medicine Research Group, and all the participants in this study.
REFERENCES
- Yuliwulandari R, Sachrowardi Q, Nishida N, Takasu M, Batubara L, Susmiarsih TP, et al. Polymorphisms of promoter and coding regions of the arylamine N-acetyltransferase 2 (NAT2) gene in the Indonesian population: proposal for a new nomenclature. J Hum Genet. 2008;53(3):201–9.
- García-Martín E. Interethnic and intraethnic variability of NAT2 single nucleotide polymorphisms. Curr Drug Metab. 2008;9(6):487–97.
- McDonagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for N-acetyltransferase 2. Pharmacogenet Genomics. 2014;24(8):409–25.
- Fernandes MR, de Carvalho DC, dos Santos ÂK, dos Santos SE, de Assumpção PP, Burbano RM, et al. Association of slow acetylation profile of NAT2 with breast and gastric cancer risk in Brazil. Anticancer Res. 2013;33(9):3683–9.
- Yuliwulandari R, Susilowati RW, Wicaksono BD, Viyati K, Prayuni K, Razari I, et al. NAT2 variants are associated with drug-induced liver injury caused by anti-tuberculosis drugs in Indonesian patients with tuberculosis. J Hum Genet. 2016;61(6):533–7.
- Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PLoS One. 2011;6(4):e18507.
- Ananta A, Arifin E, Hasbullah MS, Handayani NB, Pramono A. Changing ethnic composition in Indonesia, 2000-2010. XXVII IUSSP International Population Conference. 2013:1–32. Available from: https:// iussp.org/sites/default/files/event_call_for_papers/ IUSSP%20Ethnicity%20Indonesia%20Poster%20 Section%20G%202708%202013%20revised.pdf
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–89.
- Sabbagh A, Darlu P. Inferring haplotypes at the NAT2 locus: the computational approach. BMC Genet. 2005;6:30.
- Sabbagh A, Langaney A, Darlu P, Gérard N, Krishnamoorthy R, Poloni ES. Worldwide distribution of NAT2 diversity: Implications for NAT2 evolutionary history. BMC Genet. 2008;9:21.
- Selinski S, Blaszkewicz M, Ickstadt K, Hengstler JG, Golka K. Refinement of the prediction of N-acetyltransferase 2 (NAT2) phenotypes with respect to enzyme activity and urinary bladder cancer risk. Arch Toxicol. 2013;87(12):2129–39.
- Du H, Chen X, Fang Y, Yan O, Xu H, Li L, et al. Slow N-acetyltransferase 2 genotype contributes to antituberculosis drug-induced hepatotoxicity: a metaanalysis. Mol Biol Rep. 2013;40(5):3591–6.
- Gong C, Hu X, Gao Y, Cao Y, Gao F, Mo Z. A meta-analysis of the NAT1 and NAT2 polymorphisms and prostate cancer: a huge review. Med Oncol. 2011;28(1):365–76.
- Unal M, Tamer L, Doğruer ZN, Yildirim H, Vayisoğlu Y, Camdeviren H. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope. 2005;115(12):2238–41.
- Van Eyken E, Van Camp G, Fransen E, Topsakal V, Hendrickx JJ, Demeester K, et al. Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J Med Genet. 2007;44(9):570–8.
- Bared A, Ouyang X, Angeli S, Du LL, Hoang K, Yan D, et al. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol Head Neck Surg. 2010;143(2):263–8.
- Kosova B, Çetintaş VB, Çal AÇ, Tetik A, Özel R, Aktan Ç, et al. N-acetyltransferase 2 gene polymorphisms and susceptibility to prostate cancer : a pilot study in the Turkish population. Turk J Med Sci. 2010;40(4):629–36.
- Liu HE, Hsiao PY, Lee CC, Lee JA, Chen HY. NAT2*7 allele is a potential risk factor for adult brain tumors in Taiwanese population. Cancer Epidemiol Biomarkers Prev. 2008;17(3):661–5.
- Moore LE, Baris DR, Figueroa JD, Garcia-Closas M, Karagas MR, Schwenn MR, et al. GSTM1 null and NAT2 slow acetylation genotypes, smoking intensity and bladder cancer risk: results from the New England bladder cancer study and NAT2 meta-analysis. Carcinogenesis. 2011;32(2):182–9.
- Figueroa JD, Han SS, Garcia-Closas M, Baris D, Jacobs EJ, Kogevinas M, et al. Genome-wide interaction study of smoking and bladder cancer risk. Carcinogenesis. 2014;35(8):1737–44.
- Ruiz JD, Martínez C, Anderson K, Gross M, Lang NP, García- Martín E, et al. The differential effect of NAT2 variant alleles permits refinement in phenotype inference and identifies a very slow acetylation genotype. PLoS One. 2012;7(9):e44629.
- Selinski S, Blaszkewicz M, Getzmann S, Golka K. N-Acetyltransferase 2: ultra-slow acetylators enter the stage. Arch Toxicol. 2015;89(12):2445–7.
- An HR, Wu XQ, Wang ZY, Zhang JX, Liang Y. NAT2 and CYP2E1 polymorphisms associated with antituberculosis drug-induced hepatotoxicity in Chinese patients. Clin Exp Pharmacol Physiol. 2012;39(6):535–43.
- Selinski S, Getzmann S, Gajewski PD, Blaszkewicz M, Hengstler JG, Falkenstein M, et al. The ultra-slow NAT2*6A haplotype is associated with reduced higher cognitive functions in an elderly study group. Arch Toxicol. 2015;89(12):2291–303.
- Ng CS, Hasnat A, Al Maruf A, Ahmed MU, Pirmohamed M, Day CP, et al. N-acetyltransferase 2 (NAT2) genotype as a risk factor for development of drug-induced liver injury relating to antituberculosis drug treatment in a mixed-ethnicity patient group. Eur J Clin Pharmacol. 2014;70(9):1079–86.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id