
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
The low prevalence of colonic serrated adenocarcinoma with high KRAS mutational status at Cipto Mangunkusumo Hospital, Indonesia
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v27i3.1719Med J Indones. 2018;27:161–8
Received: December 14, 2016
Accepted: August 16, 2018
Author affiliation:
Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Corresponding author:
Nur Rahadiani
E-mail: nur.rahadiani@ui.ac.id
Background
Serrated adenocarcinoma (SA), a subtype of colorectal carcinoma, and the KRAS mutation, a strong marker for the patient’s response to anti-epidermal growth factor receptor therapy, have a clinical importance because of its progressive nature and tendency for chemoresistance. The purposes of this study were to (1) determine the prevalence of SA, (2) evaluate the histomorphological characteristics of SA and classical adenocarcinoma based on its prognostic factors, (3) determine the prevalence of the KRAS mutation in SA cases, and (4) identify the main characteristics of SA cases and classical adenocarcinoma with a KRAS mutation.
Methods
This study was conducted by reviewing hematoxylin-eosin-stained slides of colorectal carcinoma (CRC) cases from January 2013 to July 2015 at the Department of Anatomical Pathology Cipto Mangunkusumo General Hospital. The final diagnosis of SA was based on the Tuppurainen et al criteria and the KRAS mutation was evaluated using real-time polymerase chain reaction.
Results
Among the 117 adenocarcinoma cases, there were 41 unequivocal SA, 11 equivocal SA, and 65 classical adenocarcinoma. The prevalence rates of unequivocal and equivocal SA among all CRC cases were 7.7% and 2.1%, respectively. There were 11 (28.2%) cases of wild-type KRAS and 28 (71.7%) cases of mutated KRAS among all unequivocal SA cases. Tumor budding (TB) was the predominant prognostic factor
Conclusion
The prevalence of SA among all CRC cases was 7.7%. The KRAS mutation was found in almost three-quarters of all SA cases.
Keywords
colon cancer, KRAS mutation, serrated adenocarcinoma
The 2014 World Cancer Report revealed that colorectal carcinoma (CRC) is the third most common cancer in the world and contributes to 10% of the global burden of cancer. Furthermore, CRC is the fourth most prevalent cause of cancerrelated deaths regardless of gender.1–3 A similar epidemiological pattern is seen in Indonesia, where CRC prevalence has been steadily increasing over the past decade and most recently made up approximately 10% of the total number of cancers, as reported by The Indonesian Cancer Registration Agency.4
The most common histological type of CRC is adenocarcinoma, constituting 80% of all CRC cases. This type is known for its invasive nature and high distant metastasis rate.5 Based on the 2010 World Health Organization (WHO) classification, adenocarcinoma of the colon is divided into seven histological subtypes: (1) adenocarcinoma (classic type, not otherwise specified/NOS), (2) cribriform-comedo carcinoma, (3) mucinous adenocarcinoma, (4) medullary adenocarcinoma, (5) micropapillary adenocarcinoma, (6) signet ring-cell adenocarcinoma, and (7) serrated adenocarcinoma (SA).2
There has been a growing interest in studying SA over the last few years, particularly after discovery of the serrated pathway of CRC, which contributes to the rapidly progressive behavior and chemoresistance of CRC.6,7 Through mutations in KRAS and some other genes, the serrated pathway causes progressive changes in serrated polyps, including sessile serrated adenoma (SSA) and traditional serrated adenoma (TSA), into carcinoma.6 Cases of CRC that show rapid tumor growth and poor survival rates are thought to arise from this alternative pathway.7
However, diagnosing SA remains a challenge for many pathologists due to the lack of definite histomorphological diagnostic criteria.2,8 The 2010 WHO criteria state that SA histomorphology can resemble that of SSA, which is not entirely helpful since SSA is often difficult to recognize. Because of this uncertainty, many academic centers do not use SA as a routine diagnostic term. Some researchers have proposed other criteria to assist with the diagnosis of SA as reported by Tuppurainen et al.8
KRAS is an oncogene that plays an important role in the carcinogenesis of CRC. The KRAS gene produces a protein that binds guanosine triphosphate (GTP) and guanosine diphosphate (GDP). This protein is located in the inner layer of the cell membrane and functions as an “on-off” switch to regulate signal transduction of GTP and control cell growth and differentiation.9 A mutation in the KRAS gene contributes to CRC resistance to anti-epidermal growth factor receptor (anti-EGFR) targeted chemotherapeutic agents, such as panitumumab and cetuximab, making KRAS mutational status a suitable predictive marker for the therapeutic response. Using the KRAS mutational status as a guide, oncologists are more confident determining which patients with SA would be the best candidates for anti-EGFR targeted therapy.9
No data are available in Indonesia on the prevalence of SA and its KRAS mutational status. The purposes of this study were to: (1) determine the prevalence of SA, (2) evaluate the histomorphological characteristics of SA and classical adenocarcinoma based on its prognostic factors, (3) determine the prevalence of the KRAS mutation in SA cases, and (4) identify the main characteristics of SA cases and classical adenocarcinoma with the KRAS mutation.
METHODS
This study was conducted in the Department of Anatomical Pathology and the Molecular Pathology Laboratory at Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital (FMUI-CMH) from April to December 2016. Ethical clearance (no. 406/UN2.F1/ETIK/2016) was obtained from the Institutional Review Board of the Faculty of Medicine Universitas Indonesia.
We used a secondary data from tissue slides, formalin-fixed paraffin embeded (FFPE) blocks, and histopathology examination records of all colorectal adenocarcinoma cases from January 2013 to July 2015 archived at the Department of Anatomical Pathology FMUI/ CMH. Among 528 CRC cases documented from January 2013 to July 2015, 137 were adenocarcinoma. Twenty adenocarcinoma cases were excluded for the following reasons: incomplete or unavailable slides, FFPE blocks, or forms (14 cases); suspected to be metastatic adenocarcinoma (two cases); and differential diagnosis of a neuroendocrine tumor (four cases), leaving 117 eligible cases for the study. Cases obtained from biopsies must have also been excluded, yet there was none of biopsied cases met this criteria.
All eligible cases were validated by hematoxylin and eosin (HE) staining. All HEstained slides and FFPE tissue blocks were evaluated by two pathologists (NR and DRH). Using the criteria of Tuppurainen et al,8 the evaluators looked for the following eight features, i.e., epithelial serrations, eosinophilic or clear cytoplasm, abundant cytoplasm, vesicular nuclei, prominent nucleoli, the absence of tumor necrosis or tumor necrosis <10%, intra or extracellular mucin production, and cell balls or papillary rods. These cases with at least six of the first seven features were classified as “unequivocal (definite) SA”, whereas cases with five of all eight features were classified as “equivocal (probable) SA”.10 Cases that did not meet the criteria for unequivocal and equivocal SA were classified as “classic adenocarcinoma” or “adenocarcinoma NOS”.
We furthered the evaluation by examining the presence of four prognostic factors, i.e., lymphovascular invasion, perineural invasion, lymphocyte infiltration, and tumor budding (TB). KRAS mutational status was evaluated only for the unequivocal SA cases. Among the 41 identified unequivocal SA cases (Table 1), two were excluded due to unavailable FFPE blocks. The KRAS mutation was analyzed using the realtime polymerase chain reaction (RT-PCR) with the PNAClamp KRAS Mutation Detection Kit (Panagene, Seoul, Korea, cat. no. PNAC-1006). Deoxyribonucleic acid (DNA) was analyzed using DNA extracted from the FFPE block samples (Nucleospin DNA FFPE XS; Macherey-Nagel, Düren, Germany, cat. no. 740980.50) and DNA products were quantified using the NanoVue spectrophotometer.
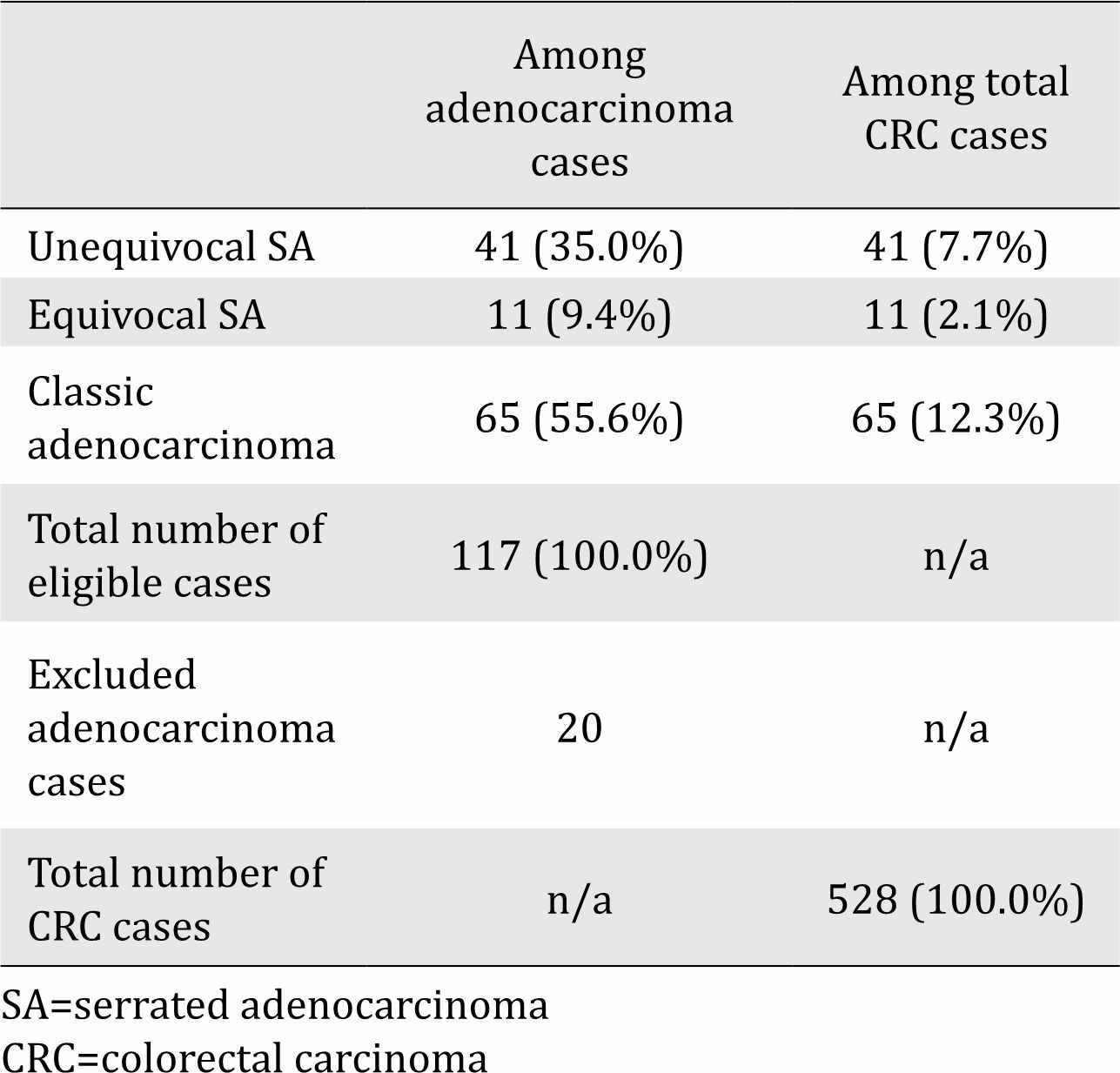
Table 1. Prevalence of serrated adenocarcinoma (SA) among adenocarcinoma and all colorectal carcinoma (CRC) cases
The polymerase chain reaction (PCR) mixture included the KRAS PNA premix, the PNA mix, and the G12PNA, G13PNA, A59PNA, Q61PNA, K117PNA, and A146PNA probes. The DNA process started by mixing the target DNA with distilled water, premix, and the probes, resulting in a 56 μL DNA mixture, as instructed by the kit. The DNA mixture was placed on a 96-well PCR plate. The plate was covered with a film applicator and centrifuged at 1,000 rpm. The PCR plate was inserted into an RT-PCR machine for 40 PCR cycles consisting of three phases, i.e., the denaturation phase at 94°C for 30 sec, the annealing phase at 63°C for the next 30 sec, and the extension phase at 72°C for the final 30 sec. The RT-PCR machine showed the cycle threshold (CT)-value results at the end of each phase and automatically transformed the results to a Microsoft Excel® spreadsheet format.
The histopathology records provided the information about age, gender, tumor location, and tumor characteristics (size, tumor growth, differentiation, pT stage, lymphovascular and perineural invasion, lymph node involvement and metastasis, TB, and lymphocyte infiltration) of each case. Based on this information, we identified the characteristics of the SA cases and the KRAS mutation being studied.
Data processing and analysis were performed with the Statistical Package for Social Sciences (SPSS)® version 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to calculate the prevalence rates of SA and the KRAS mutation. The four prognostic factors and demographic characteristics of the SA cases were also analyzed for their frequency distributions and associations with SA prevalence using the chi-square and Fisher’s exact test. A p-value <0.05 was considered significant.
RESULTS
Among the 117 adenocarcinoma cases studied, more than half (55.6%) were the classic subtype, about one-third (35.0%) were unequivocal SA, and the rest (9.4%) were equivocal SA (Table 1). The prevalence rates of unequivocal SA and equivocal SA among all CRC cases were 7.7% and 2.1%, respectively.
Table 2 shows the relatively similar characteristics between unequivocal SA in general and cases with the KRAS mutation, except there was no lymph node involvement in unequivocal SA cases with the mutant KRAS gene. Unequivocal SA was more frequent among adults ≥40 years and was more commonly found in the left colon. The majority of the tumors were >5 cm in diameter with a well differentiated exophytic tumor growth pattern. The most common pathological tumor extension grades were T3 and T4 with N1 and N2 lymph node involvement and no metastasis.
Table 2. Prognostic factors among adenocarcinoma and KRAS mutation among unequivocal serrated adenocarcinoma (SA) cases
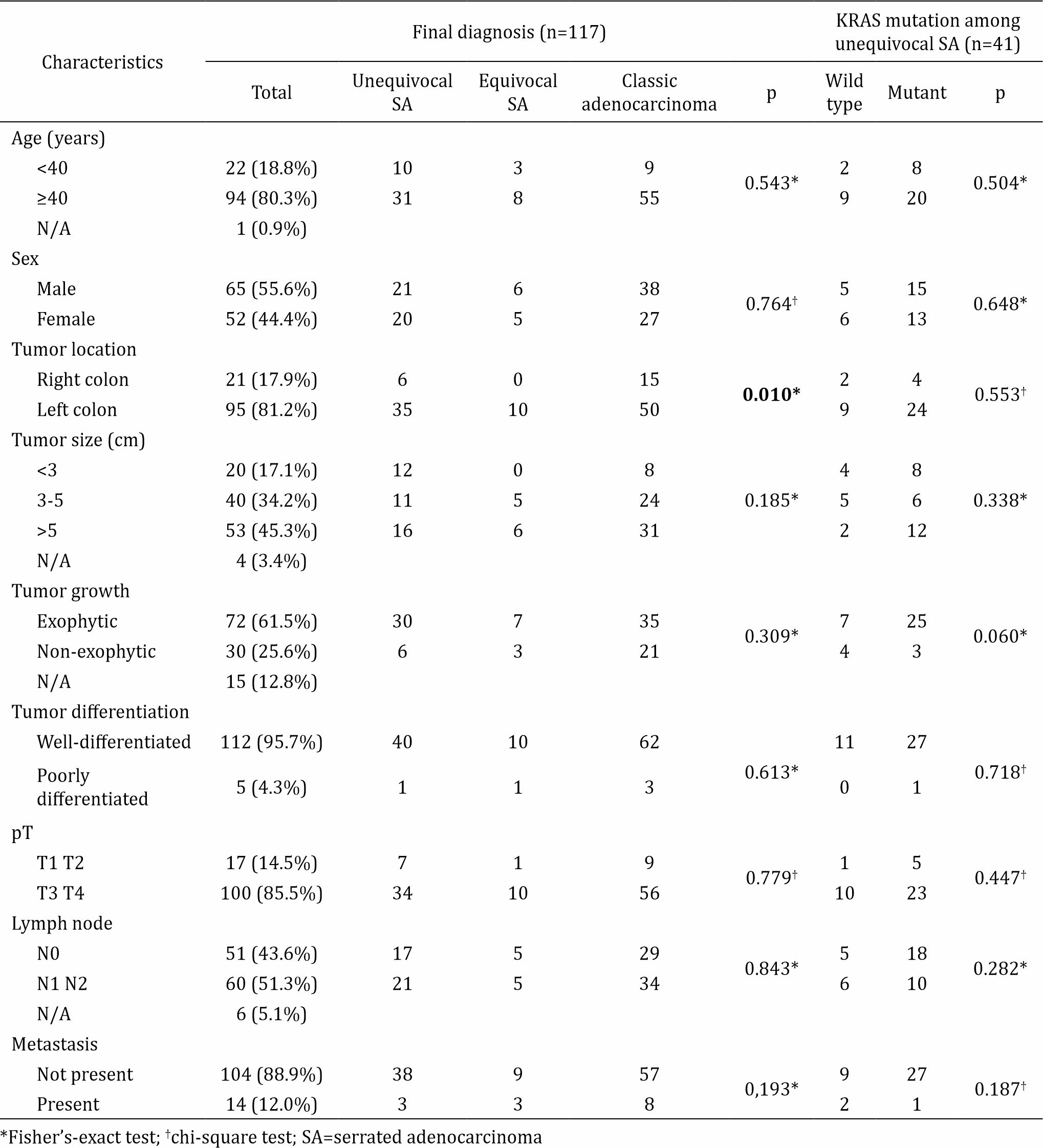
Table 3. Prevalence of the KRAS mutation among unequivocal serrated adenocarcinoma (SA) cases (n=39)
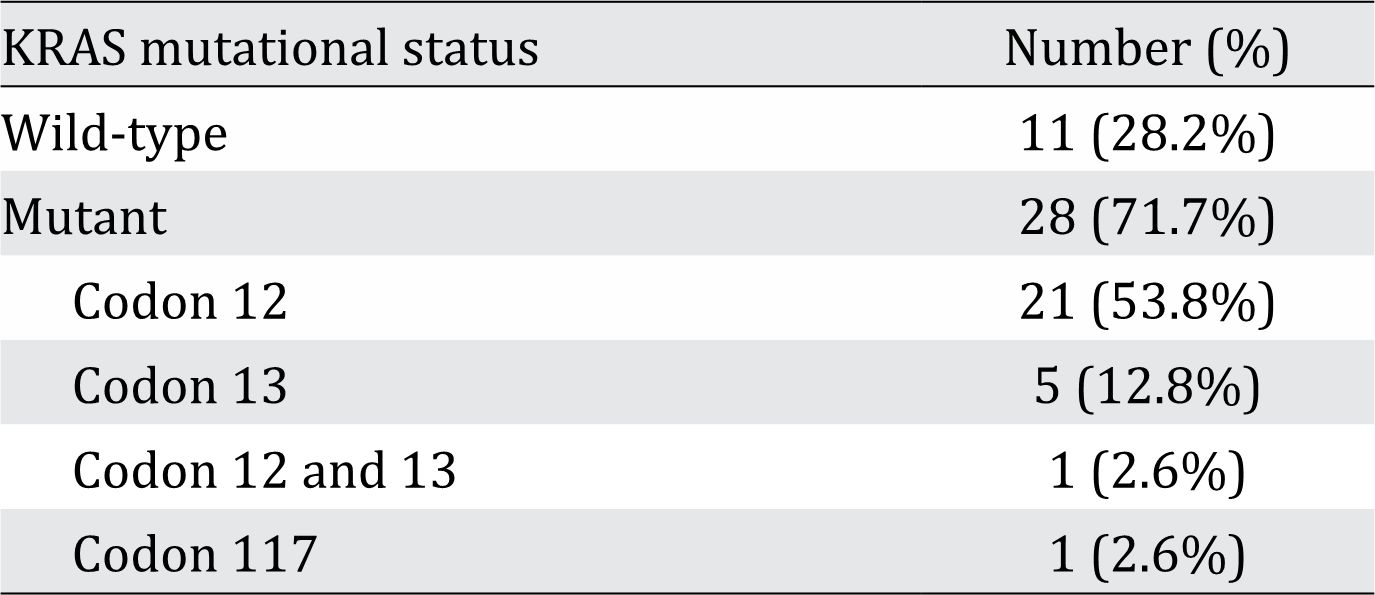
Table 2 also shows the distribution of prognostic factors among all adenocarcinoma cases. Mild lymphocyte infiltration was significantly more likely to occur in adenocarcinoma regardless of the subtype. TB was the most predominant prognostic factor found in unequivocal SA (32 of 41 cases). As shown in Table 3, there were 28 (71.7%) cases of unequivocal SA with the KRAS gene mutation. The two most common locations for this mutation were codon 12 (53.8%) and codon 13 (12.8%). Histomorphological appearance of serrated adenocarcinoma (SA) case and its prognostic factors can be seen in figure 1, figure 2, and figure 3.
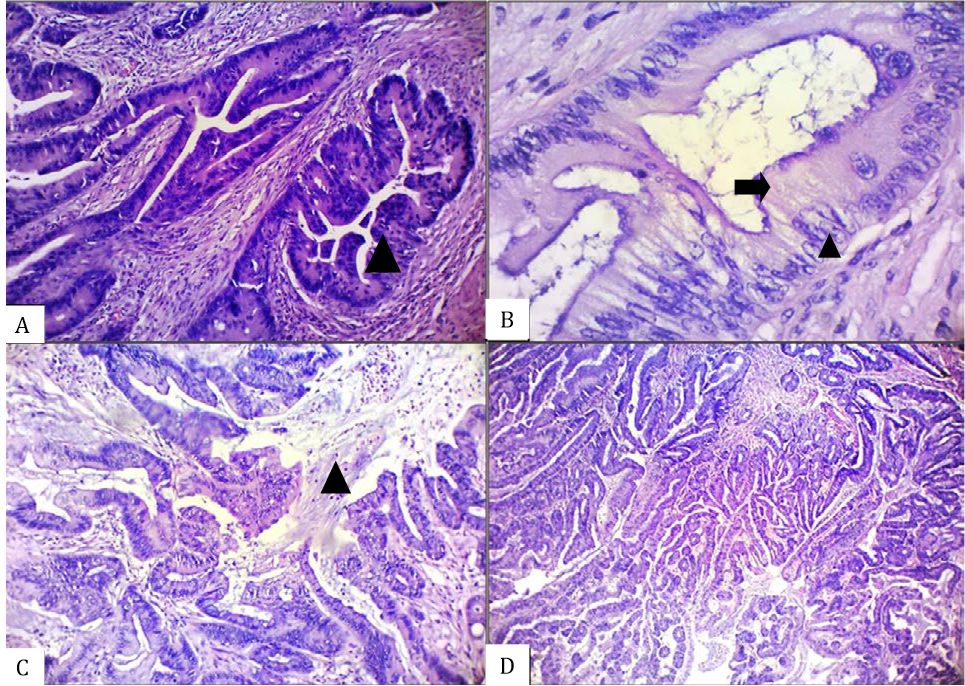
Figure 1. Histomorphological appearance of an unequivocal serrated adenocarcinoma (SA) case showing the presence of A) epithelial serrations (arrow head), B) abundant cytoplasm (arrow) with vesicular nuclei and distinct nucleoli (arrow head), C) intracellular and extracellular mucin (arrow head), and D) absence of necrosis [A–C, hematoxylin and eosin (HE), 100×. D, HE, 40×]
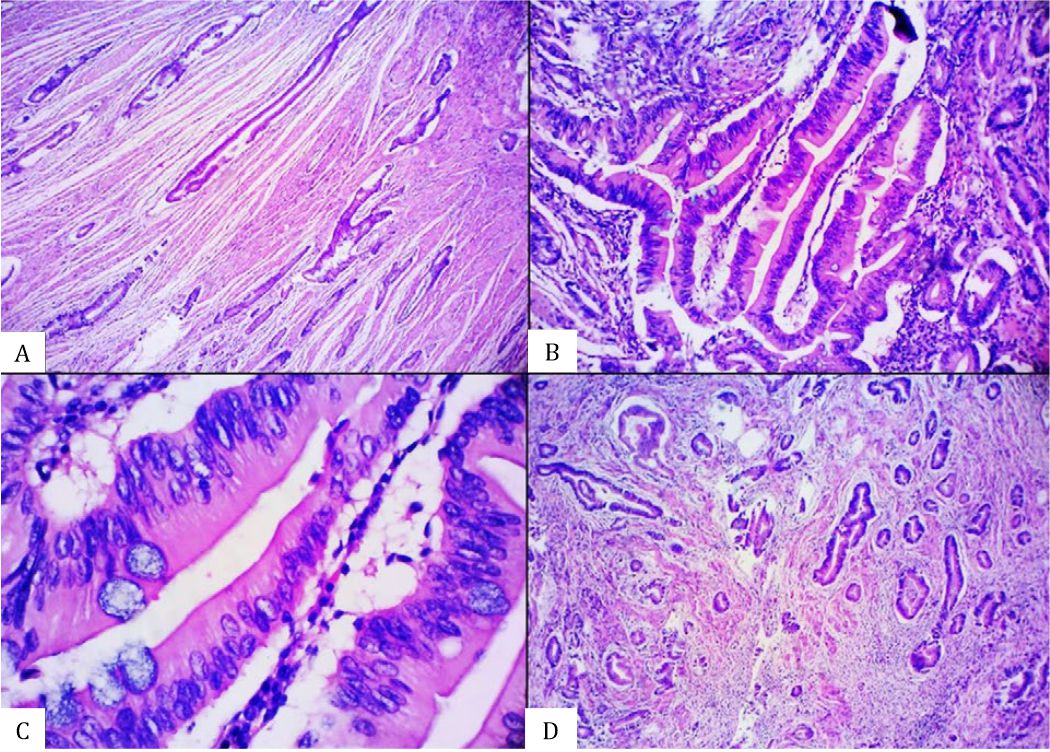
Figure 2. Histomorphological appearance of an equivocal serrated adenocarcinoma (SA) case showing the presence of A) glandular structure without epithelial serrations, B) abundant-eosinophilic cytoplasm, C) vesicular nuclei without distinct nucleoli, and D) absence of necrosis [A&D, hematoxylin and eosin (HE), 40×. B&C, HE, 100×].
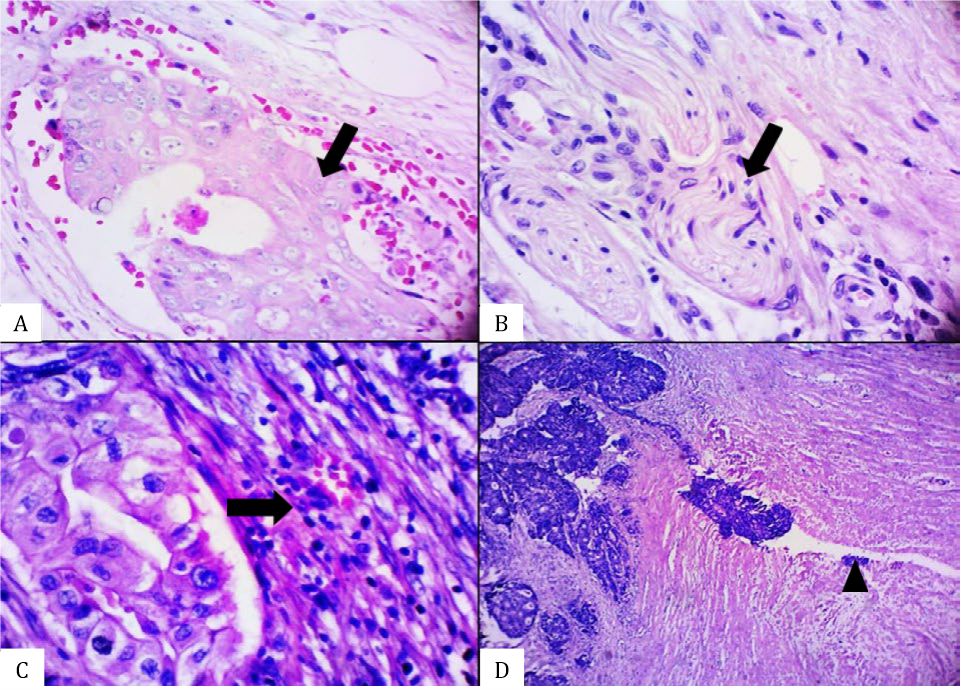
Figure 3. Histomorphological appearance of an equivocal serrated adenocarcinoma (SA) case showing the presence of A) glandular structure without epithelial serrations, B) abundant-eosinophilic cytoplasm, C) vesicular nuclei without distinct nucleoli, and D) absence of necrosis [A&D, hematoxylin and eosin (HE), 40×. B&C, HE, 100×].
DISCUSSION
SA results from malignant transformation of serrated polyps, such as SSA and TSA, through the serrated pathway.11,12 This type of carcinoma was first introduced by Jass et al13 followed by the proposed morphological criteria of Tuppurainen et al8 and was finally recognized by the WHO as colon adenocarcinoma in 2010.2 About 7.7% and 2.1% of cases were diagnosed as unequivocal and equivocal SA among all CRC cases, respectively. Although the SA diagnosis was based solely on histomorphological criteria, the prevalence of SA was relatively similar to the global prevalence, which was 7%–17.5% of overall CRC cases in 2007.7,8 This finding suggests that the histomorphological criteria may be sufficient to form a working diagnosis of SA when more definitive tools are not available, such as in remote and resource-limited areas. Some studies suggested the use of histomorphological criteria to diagnose SA.7,8,10 Adequate knowledge of the serrated morphology in a CRC specimen helps a pathologist inform the surgeon about the likelihood of rapid spread of the disease and its tendency to be chemoresistant.6,7
Clinical characteristics have no significant association with KRAS mutational status. A similar study by Kim et al14 reported a KRAS mutation prevalence of 79% among tubulovilous adenoma cases, which is slightly higher than our result (71.7% of all SA cases). However, our result was considerably higher (71.7%) when compared to the prevalence among all TSA cases (56%). We suggest that the KRAS mutant cases in our study may have originated from TSAs tubulovilous (conventional) adenomas.
KRAS mutational status is important to predict the patient’s response to anti-EGFR chemotherapy. The KRAS protein is a component of the mitogen-activated protein kinase (MAPK) pathway. When activated, this oncogene stimulates cell division and inhibits apoptosis. Ligands such as EGF and transforming growth factor (TGF) bind receptor tyrosine kinase (RTKs) and the receptor undergoes a conformational change resulting in phosphorylation of the tyrosine residue producing GTP. KRAS forms GTP bound (“on” state) which results in activation of the MAPK pathway. Epidermal growth factor receptor inhibitors (EGFR-I), such as cetuximab and panitumumab, have been approved for treatment of CRC by converting KRAS into GDP bound (“off” state), which inhibits a downstream signaling.10 A KRAS mutation results in the constitutively GTP bound conformation of the KRAS protein leading to activation of the MAPK pathway in the presence of EGFR-I.10,15 This study showed the KRAS mutational profile among CRC cases. This finding is important to propose a better treatment option for patients using targeted therapy via anti-EGFR regiments.
Pre-cancerous lesions of the colon such as SSA, TSA, and hyperplastic polyps may lead to SA. These lesions occur in young adults and in older populations. Malignant transformation of these lesions is thought to occur progressively and clinically manifest in older patients aged >50 years, as also found in the present study.16 Thus, CRC and SA should be periodically screened for starting at the age of 50 years using the fecal occult blood test and colonoscopy.16 Our findings re-emphasize the urgency of developing a nationwide CRC screening program.
SA is more common in women than men, while the incidence of CRC is relatively equal between the sexes.8,17 The reason women are more prone to malignant transformation remains controversial. It was presumably related to a folate depletion and a decrease in estrogen levels. These conditions can trigger a microsatellite instability (MSI) and a CpG methylation, which can later induce a malignant transformation in the colon7 Therefore, serrated adenomas (SAs) appearing in women during childbearing age have a higher risk of transformation into malignancy later in life compared to adenomas occurring in men of the same age. This finding suggests a complete removal surgery and a close follow-up for SA cases in women.
SAs appear in two main locations, such as the cecum (right/proximal colon) and the rectum (left/distal colon). Although SA can be found both in the right and left colon, its incidence is higher in the right colon because it is harder to reach by endoscopy. This anatomically secluded location allows polyps to be undetected or untreated and, hence, transform into a carcinoma. In contrast, polyps in the left colon are usually easier to detect and removed by surgery.
SA in the right colon generally arises from SSA, whereas SA in the left colon mainly originates from TSA.8 SSA, sporadic CRC with microsatellite instability-high (MSI-H), and SA with the MSI-H status are usually found in the right colon. In contrast, TSA, CRC with microsatellite instability-low (MSI-L), and SA with MSI-L usually occur in the left colon.7 These subtype-specific predilection sites were also seen in this study and showed a significant association between location of the tumor and the SA diagnosis (Table 2). The higher prevalence of unequivocal and equivocal SA in the left colon suggests that most SA cases in this study may have resulted from precancerous TSA lesions. The original precancerous lesion of SA can be identified by conducting a morphological assessment to clearly differentiate TSA from SSA. However, such assessment was not part of our investigation.
In this study, TB was significantly associated with SA. TB involves isolated tumor cells or small clumps of tumor with less than five cell-members per group located at the invasive part of the tumor. TB has been reported as a strong prognostic factor in classic as well as SA, and a higher degree correlates with a worse clinical outcome.18,19
The limitation of this study is that the diagnosis of SA was made only through histomorphological appearance. Immunohistochemical staining (Annexin 10) should be used to confirm these findings whenever facilities and antibody reagents are available. Immunohistochemical staining can be used as an additional examination in doubtful cases, particularly for equivocal SAs.20
In conclusion, the prevalence of SA in this study was 7.7% among all CRC cases, and TB was the most predominant prognostic factor. The KRAS mutation was a strong marker for the patient’s response to anti-EGFR therapy and was found in 71.7% of SA cases.
Conflicts of Interest
The authors affirm no conflicts of interest in this study.
Acknowledgment
We thank Dr. David Sitinjak for his contribution to the statistical analysis of this study.
REFERENCES
- Peto R, Kramer BS, Mel Greaves, Hausen H, Victoria CG, Blackburn E, et al. The global and regional burden of cancer. In: Stewart BW, Christopher P Wild, editors. World cancer report 2014. Lyon: IARC Press; 2014. p. 16.
- Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura S-I, et al. Carcinoma of colon and rectum. In: Bosman F, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010. p. 131–46.
- Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–41.
- Badan Registrasi Kanker Perhimpunan Dokter Spesialis Patologi Anatomi. Kanker di Indonesia tahun 2010: data histopatologik. Jakarta: Direktorat Jenderal Pelayanan Medik Departemen Kesehatan RI; 2010.
- Fleming M, Ravula S, Tatischev SF, Wang HL. Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol. 2012;3(3):153–73.
- Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–35.
- Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50(1):131–50.
- Tuppurainen K, Mäkinen JM, Junttila O, Liakka A, Kyllönen AP, Tuominen H, et al. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005;207(3):285–94.
- Siddiqui AD, Piperdi B. KRAS mutation in colon cancer: a marker of resistance to EGFR-I therapy. Ann Surg Oncol. 2010;17(4):1168–76.
- Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62(3):367–86.
- Lee CT, Huang YC, Hung LY, Chow NH, Su PF, Ho CL, et al. Serrated adenocarcinoma morphology in colorectal mucinous adenocarcinoma is associated with improved patient survival. Oncotarget. 2017;8(21):35165–75.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42(1):1–10.
- Jass JR, Smith M. Sialic acid and epithelial differentiation in colorectal polyps and cancer--a morphological, mucin and lectin histochemical study. Pathology. 1992:24(4):233–42.
- Kim KM, Lee EJ, Ha S, Kang SY, Jang KT, Park CK, et al. Molecular features of colorectal hyperplastic polyps and sessile serrated adenoma/polyps from Korea. Am J Surg Pathol 2011;35(9):1274–86.
- Murcia O, Juárez M, Hernández-Illán E, Egoavil C, Giner-Calabuig M, Rodríguez-Soler M, et al. Serrated colorectal cancer: molecular classification, prognosis, and response to chemotherapy. World J Gastroenterol. 2016;22(13):3516–30.
- U.S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. AHRQ Publication 08- 05124-EF-3. Rockville, USA; Agency for Healthcare Research and Quality: 2008.
- García-Solano J, Pérez-Guillermo M, Conesa-Zamora P, Acosa-Ortega J, Trujillo-Santos J, Cerezuela-Fuentes P, et al. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subsets of colorectal carcinoma. Hum Pathol. 2010;41(10):1359–68.
- Wang LM, Kevans D, Mulcahy H, O’Sullivan J, Fennelly D, Hylland J, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009;33(1):134–41.
- García-Solano J, Conesa-Zamora P, Trujillo-Santos J, Mäkinen MJ, Pérez-Guillermo M. Tumor budding and other prognostic pathological features at invasive margins in serrated colorectal adenocarcinoma: a comparative study with conventional carcinoma. Histopathology. 2011;59(6):1046–56.
- Sajanti SA, Väyrynen J, Sirniö P, Klinturp K, Mäkelä J, Tuomisto A, et al. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch. 2015;466(1):5–12.
Copyright @ 2018 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id