
Section Abstract Introduction Methods Results Discussion Conflict Of Interest Acknowledgment References
Basic Medical Research
Adipose derived stem cell conditioned medium effect on proliferation phase of wound healing in Sprague Dawley rat
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v26i4.1755 Med J Indones. 2017;26:239–45
Received: January 3, 2017
Accepted: November 15, 2017
Author affiliation:
1 Department of Histology, Faculty of Medicine, Universitas Tarumanagara, Jakarta, Indonesia
2 Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Stem Cell and Tissue Engineering Research Center, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
4 Department of Surgery, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
5 Stem Cell Medical Technology, Integrated Service Unit, Dr. Cipto Mangunkusumo General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Jeanne A. Pawitan
E-mail: jeanneadiwip@gmail.com
Background
Disintegration of skin tissue can lead to disability and death. Recent studies on wound therapy applied stem cells and adipose derived stem cell conditioned medium (ADSC-CM) to improve wound healing. However, the role of ADSC-CM in wound healing mechanism in terms of angiogenesis, quantity of collagen, and epithelialization is not fully understood. Therefore, this study aimed to analyze the levels of growth factors (VEGF and EGF) in ADSC-CM and histological features of angiogenesis, epithelialization, and collagen density after skin incision in Sprague Dawley rats.
Methods
Thirty rats were injured at the back (full thickness wound) and treated topically with ADSC-CM, culture medium, basal medium, and without treatment. Mice were sacrificed on days 3, 7, 14, 21 and 28. After sacrificed, tissue samples were examined microscopically to assess angiogenesis, epithelialization, and collagen density. Concentrations of VEGF and EGF in ADSC-CM were measured by ELISA.
Results
Clinically, wound that was treated with ADSC-CM showed improvement in wound healing process. ADSC-CM treated wound showed the highest epithelialization ratio and the fastest wound closure.
Conclusion
There were no statistical significant differences between groups that were treated with ADSCCM and not. However, topical ADSC-CM treated wound revealed a better clinical improvement in epithelialization.
Keywords
ADSC-CM, angiogenesis, collagen density, epithelialization, wound healing
Skin is the largest organ in human body with a main function as a protector from external environment. The loss of skin integrity due to injury and illness may cost disability and mortality.1 The prevalence of injuries nationwide in Indonesia by Riskesdas 2013 was 8.2%, and the major causes were falling down and traffic accident. There was an increase of injury prevalence in 2013 compared to 2007 according to Riskesdas. Abrasion was the third main injury that was experienced by the population.2
Wound healing is a series of stimulating and inhibiting processes such as cellular proliferation, differentiation, migration, and adhesion.3 This series of events involves coordination of a variety of cells, growth factors, and cytokines.3,4 Disruption of this coordination may inhibit wound healing.4
Wound healing is divided into 4 phases: hemostasis, inflammation, proliferation, and remodelling. In hemostasis phase, there are vasoconstriction and platelet aggregation in wound area. This phase is followed by inflammation that is marked by the presence of neutrophils, monocytes, and lymphocytes. Proliferation phase is an epithelialization of wound area, angiogenesis, and formation of collagen to replace fibrin that is formed at the stage of hemostasis. The last step of wound healing is remodelling and maturation of collagen in the healing tissue.5
A proper vascularization is very important to supply nutrients and oxygen to wound area in order to optimize wound healing. Angiogenesis is initiated by fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), transforming growth factor α (TGF-α), transforming growth factor β (TGF-β), and angiogenin. VEGF is a major mediator of angiogenesis to stimulate migration and proliferation of endotelial cells.6 Epithelialization is a regeneration of epidermis that is stimulated by epidermal growth factor (EGF), PDGF, FGF-2 and TGF-β. The migration of epithelial cells will close the wound and followed by proliferation and differentiation of epithelial cells to form epidermis layer.1 Collagen is a key component in providing strength to tissues. Collagen fibers are synthesized by fibroblasts and play a role in the wound closure.7 The density of collagen can be assessed in histological specimens with special staining.
The application of stem cell culture derived conditioned medium as a therapy has been studied both in vitro and in vivo with excellent results. Therapy using conditioned medium is more promising than the stem cell itself due to the ease of production, packaging and distribution.8 Previous in vitro study showed that adipose derived stem cell conditioned medium (ADSC-CM) could increase cell migration of keratinocytes, fibroblasts, and endothelial cells. Improving cell migration is expected to enhance angiogenesis, epithelialization, and fibroplasia through paracrine role of adipose derived stem cells (ADSC).9,10
The role of ADSC-CM in the in vivo process of angiogenesis, collagen density, and epithelialization are little known. Therefore, the aim of this study was to analyze the levels of growth factors (VEGF and EGF) in ADSC-CM and histological assessment of three processes (angiogenesis, epithelialization, and collagen density) after application of ADSC-CM to skin incision in Sprague Dawley rats.
METHODS
This was an experimental study using Sprague Dawley rats. Aged 8–12 weeks weighing 250– 350 g. Animals were in good health during the research period and had bleeding time of less than 1 minute. Ethical clearance (No142/ UN2.F1/ETIK/2015) was obtained from Ethical Committee of the Faculty of Medicine, Universitas Indonesia.
Thirty rats received four cuts in the back area (full thickness wound) 2 cm long with a depth of 5 mm. The four cuts of each rat were treated randomly with ADSC-CM (100%), complete culture medium, basal medium, and without treatment (control). The treatment was only done once after the rat skin injury. Therefore, there were four groups based on the treatment. Six rats were sacrificed at every time point on days 3, 7, 14, 21 and 28. After the rats were sacrificed, scar tissue were examined microscopically.
ADSC-CM was obtained from Stem Cell Medical Technology Integrated Service Unit, Cipto Mangunkusumo Hospital, Faculty of Medicine, Universitas Indonesia. ADSC-CM was aliquoted and stored in a freezer at -20°C until it was used. ADSC-CM was obtained from passage-3 of ADSC culture that was cultured in complete medium, which contained α-MEM (basal medium) and 10% platelet rich plasma (PRP) in normoxia condition for three days. ADSC-CM derived from cells showed the characteristics of ADSC, i.e.: positive for CD 34 (low), CD 73 (high) and CD 90 (high), and could differentiate into three cell lineages: osteogenic, chondrogenic, and adipogenic lineages.
ADSC-CM contained various beneficial factors that were secreted by the mesenchymal stem cells, i.e. various kinds of growth factors such as EGF and VEGF, cytokines and membrane bound structures such as microvesicles and exosomes that contained various proteins, peptides, mRNA, small interfering RNA, etc. Complete medium did not contain cell secreted factors, and only contain basal medium and 10% PRP, while basal medium was a component of the complete medium.
Concentration of EGF and VEGF in ADSC-CM was examined by enzyme-linked immunosorbent assay (ELISA) using EGF (RAB0149) and VEGF (RAB0507) Sigma-Aldrich Elisa kit. All ELISA assays were conducted in four repetitions.
Tissue samples were processed into parafin blocks, cut, and then stained with HE and Masson’s Trichome. The tissues were observed using a light microscope with a magnification of 40 and 400 times. Specimens were photographed by using Optilab advance plus camera and documented.
Histological evaluation was done in terms of angiogenesis, epithelialization, and collagen density. Angiogenesis was calculated on days 7, 14, 21 and 28 using a counting software on Optilab advance plus. Epithelialization was measured by epithelialization ratio and epithelialization length. Epithelialization ratio was the length of epithelial regeneration area divided by the length of the entire epithelialization area. Epithelialization length was the distances at the two edges of the wound epithelialization areas that were covered by epithelium. Epithelialization ratio and length were measured using measuring software of Optilab advance plus on days 3 and 7. The density of collagen was assessed in Masson’s Trichome stained specimens using ImageJ software on day 14 and 28.
The data obtained were in the form of numbers. Data were computed into median and minimummaximum or mean and standard deviation when appropriate, and presented in tables and bar diagram. Data were analyzed by analysis of variance (ANOVA) when they were appropriate for parametric test, or otherwise by non parametric test (Kruskal-Wallis) using Statistical Package for the Social Sciences (SPSS) version 22 to compare the four groups in terms of angiogenesis, epithelialization ratio and collagen density.
RESULTS
Thirty rats were injured at the beginning of the study. One rat died after wounded. The remaining 29 rats were analyzed until the end of the study. The concentrations (means ± standard deviations) of VEGF and EGF in ADSC-CM assessed by ELISA were 5052.7±0.3pg/mL and 0.2±0.1 pg/mL respectively.
Angiogenesis was analyzed on days 7, 14, 21 and 28. Figure 1 shows angiogenesis assessment on day 7 (A) and day 14 (B). Kruskal-Wallis analysis to compare the four groups in terms of angiogenesis showed that p values were higher than 0.05 (p day 7=0.547; p day 14=0.727; p day 21=0.796; p day 28=0.993) (Figure 1).
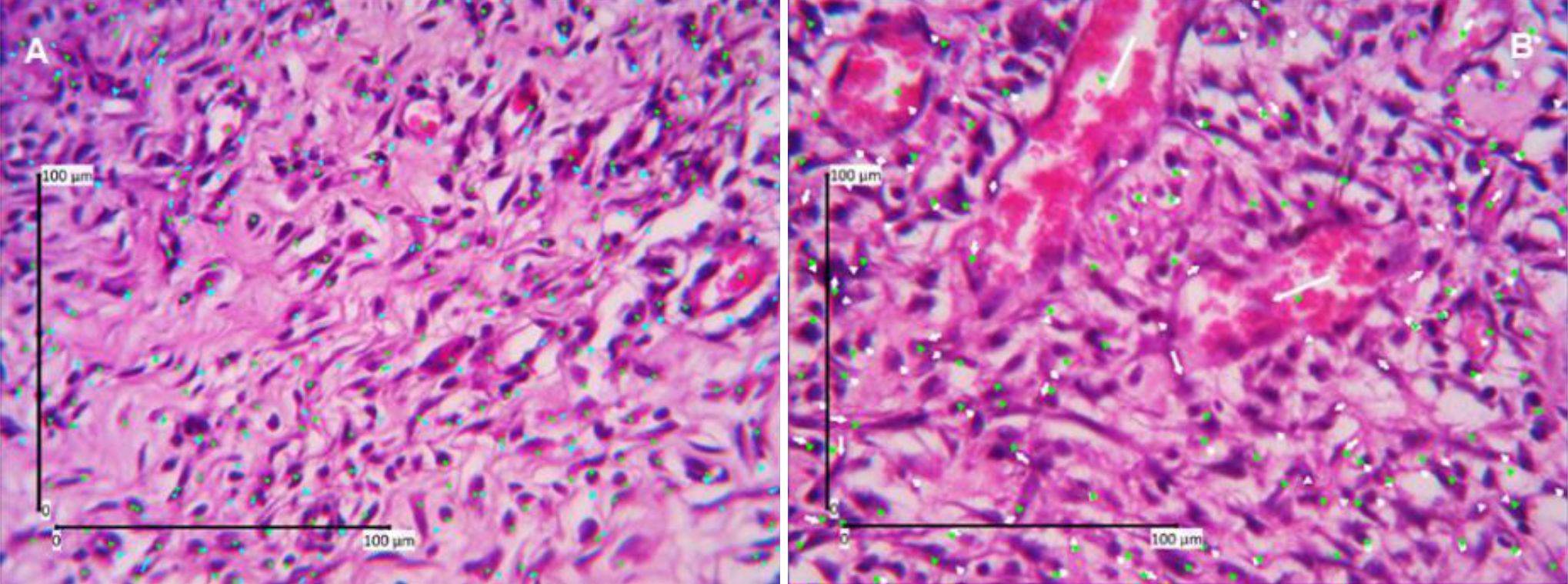
Figure 1. Angiogenesis assessment (HE staining, 400 times magnification). A) Day-7; B) Day-14, green dots show the calculation results of assessors one, blue and white dots show the calculation results of assessors two.
Epithelialization was assessed by calculating the length and the ratio of epithelialization on day 3 and day 7. Figure 2 shows assessment of epithelialization ratio. Our study found a wound with different behavior to other wounds i.e. a late epithelialization and wound gap widening compared to the previous day. Kruskal-Wallis test results in comparing the four groups on both parameters all showed that p values were higher than 0.05 (Figure 2).
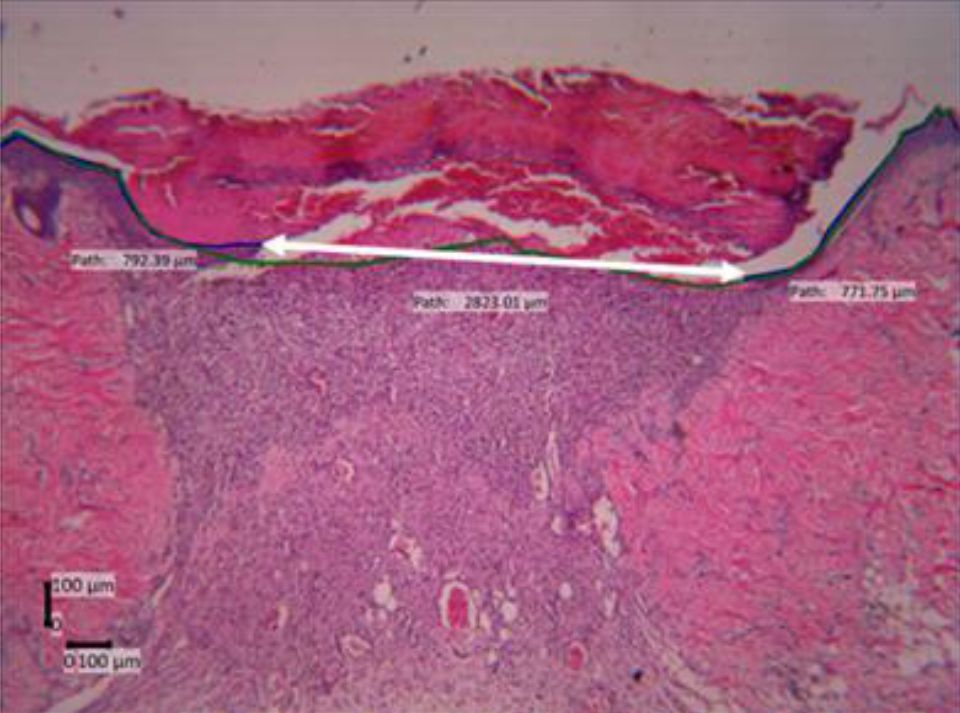
Figure 2. Epithelialization length and ratio assessment (day- 7, HE staining, 40 times magnification). Green line=the length of the whole wound area, blue line=the length of epithelial regeneration area (epithelialization length), white double headed arrow=the distance of the two edges of remaining wound, epithelialization ratio=ratio of blue/greenline
Collagen density measurements were performed on day 14 and 28. Figure 3 shows collagen density assessment. Collagen density in skin wound preparations on day 14 and day 28 can be seen in Figure 4. Kruskal-Wallis test that compared the four groups in terms of collagen density showed p values that were higher than 0.05 (p day-14=0.707, p day-28=0.513). Figure 5 summarizes the results of angiogenesis, ratio, and length of epithelialization in the four groups.
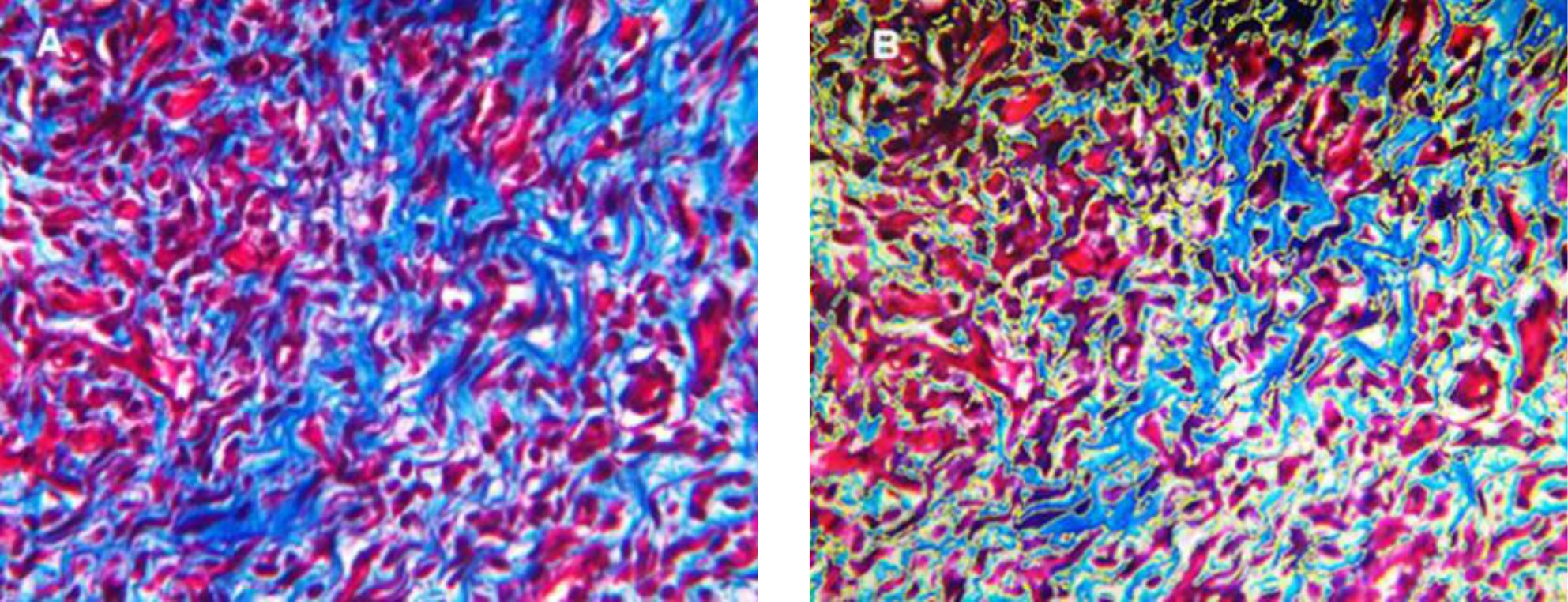
Figure 3. Collagen density assessment (Masson's Trichome staining, 400 times magnification day-14). A) original appearance; B) collagen density measurement, yellow line=demarcation of measured area that was analyzed by ImageJ
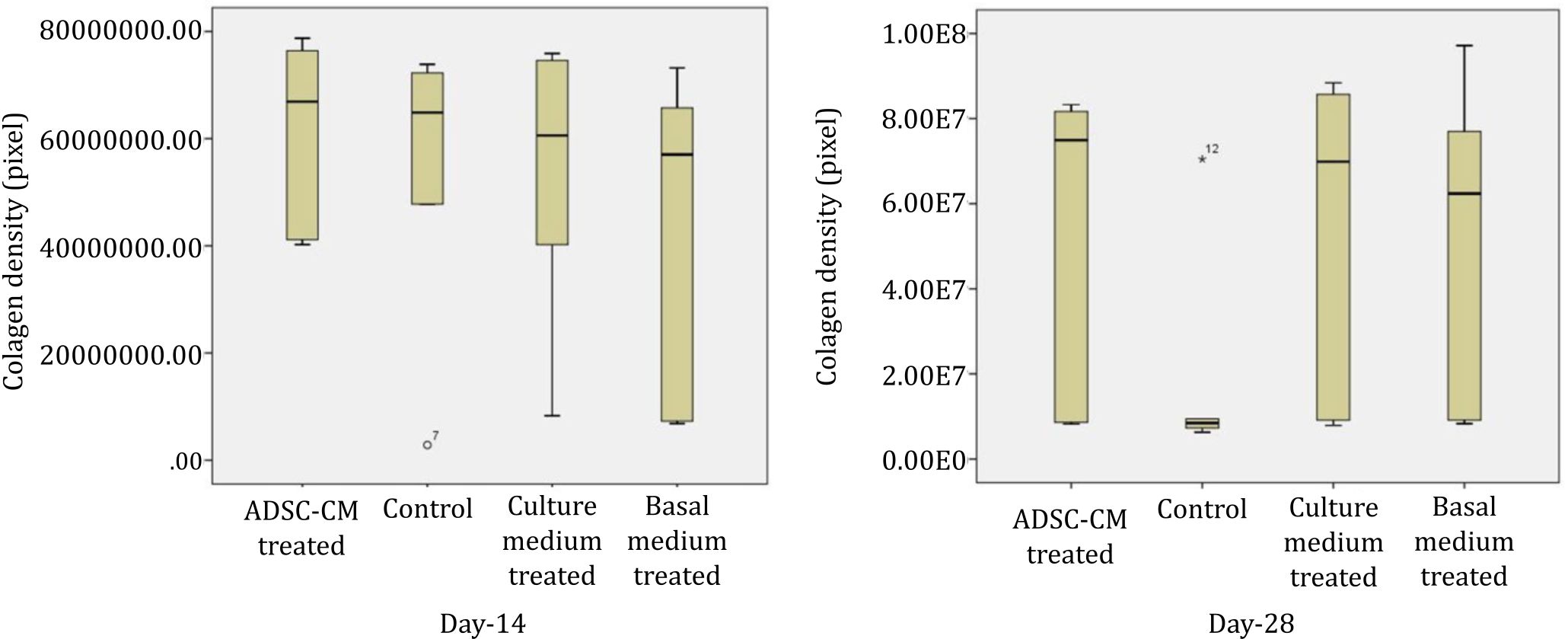
Figure 4. Collagen density in skin wound preparations on day-14 and day-28 in four groups
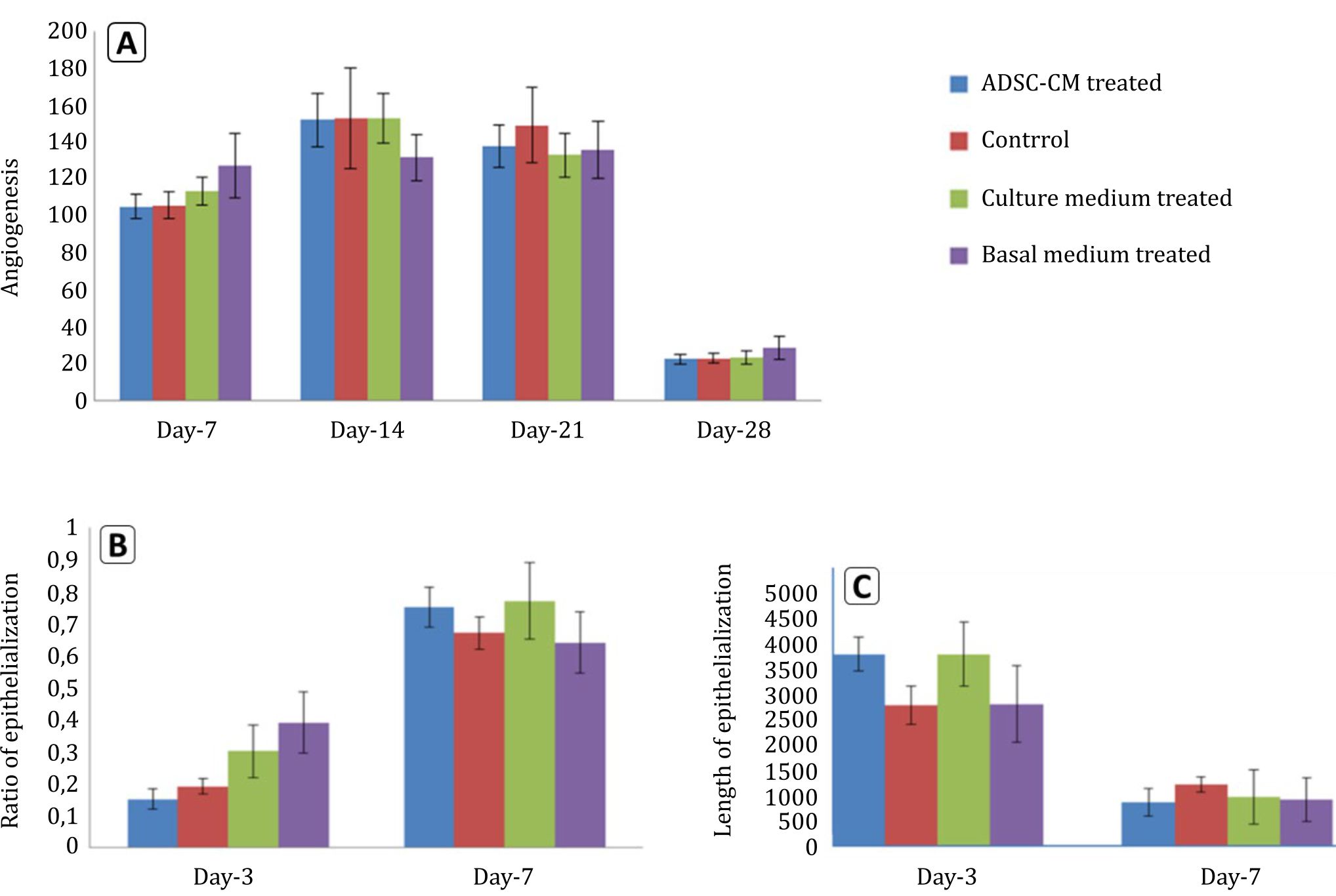
Figure 5. Angiogenesis, ratio and length of epithelialization in the four groups. A) angiogenesis; B) ratio of epithelialization; C) length of epithelialization
DISCUSSION
Our study showed that single application of ADSCCM that was produced in normoxia, and three day incubation showed no statistical significant difference compared to control wounds during proliferation phase of wound healing although ADSC-CM caused faster epithelialization. A previous study revealed that mesenchymal stem cells (MSCs) played a role in enhancing wound healing process either in intralesion injection or topical administration. Improvement of wound healing process was supposed to be a paracrine effect of MSCs rather than proliferation and differentiation of MSCs to replace damaged cells in wound tissue.10 Moreover, recent studies revealed that conditioned medium from adipose stem cell cultures contained a number of growth factors and cytokines such as VEGF, EGF, PDGF, and TGF β.8,11 VEGF is a specific mitogen for vascular endothelial cells. VEGF is a key component in the angiogenesis process of wound healing.12,13 Therefore, in this study, topical administration of ADSC-CM was expected to enhance the process of angiogenesis in rat skin wound. Futhermore, epithelialization is one of the important processes of wound healing. Skin lesion will not heal if the epithelialization does not occur. This process is dominated by migration, proliferation, and differentiation into keratinosit.14,15 Molecules that play a role in this process are EGF, FGF, and TGF-β. EGF is a key regulator of this process.16
The minimum effective concentration of VEGF to induce angiogenesis in vivo is 5000 pg/ mL.17,18 The concentrations of VEGF and EGF in our study were measured by ELISA, and the mean concentration of VEGF and EGF in our ADSCCM was 5052.7±0.3 pg/mL and 0.2±0.1 pg/mL. Compared to the result of another study by Kwon et al17 a significant difference to our study was found as their VEGF concentration was 12.3±2.4 ng/mL. Our ADSC-CM was obtained from passage-3 ADSC culture in 10% PRP containing α-MEM and normoxia condition for three days, which can be regarded as cell culture waste product. In this study, we showed that cell culture waste product contained growth factors and can be used in various conditions that need growth factors for healing. Differences of VEGF and EGF concentrations in our conditioned medium from various other studies may be due to differences in the type and the origin of cells, cell culture method, culture condition and culture medium, which in our study were adipose tissue derived stem cells, two dimension culture, normoxia and 10% PRP containing α-MEM, respectively. Some studies used spheroid cell culture method in addition to either serum or insulin supplements in the basal medium hypoxic conditions, which can stimulate cells to secrete more growth factors.11,19,20 Duration and serum-free culture medium can also influence the concentration of growth factors in ADSC-CM.8
In this study, we assessed angiogenesis, collagen density, ratio and the length of epithelialization in histological specimens. Statistical analysis showed no significant differences between ADSCCM treated wounds and others, namely the control group, culture medium group, and basal medium group. As VEGF concentration in our conditioned medium met the minimal concentration that was required for angiogenesis, this non-significant differences might be due to the necessity to repeat the administration of ADSC-CM or systemic effects as the treatments and control were performed in the same rat, which was the limitation of our study. ADSC-CM contains cytokines and growth factors that have a short half-life, therefore it is advisable that repetitive ADSC-CM application is given.8,21 Moreover, the non-difference between ADSC-CM and culture medium might be due to the presence of low level of growth factors from the 10% PRP.
In our study, angiogenesis was calculated based on blood vessel counting in specimens with routine HE staining. This staining poses difficulties in determining a blood vessel especially a small blood vessel when it is collapse. Moreover, most blood vessels that are found in angiogenesis are small blood vessels or buds. Therefore, the calculation might be lower than the reality. However, this limitation could be ignored as it is applied to the four groups.
In our study, clinically, ADSC-CM administration to incision wound showed the best result in parameter of epithelialization. ADSC-CM wound showed bigger ratio of epithelialization compared to control at day-7 and the fastest wound closure, which can be seen at the increase epithelialization ratio between day-3 and -7, and decrease in the length of epithelialization between day-3 and -7 (Figure 5). Our study found a wound with a late epithelialization and wound widening gap compared to the previous day. This fact might be due to stress factors in the rat. Stress factors can decrease wound healing process significantly.5 Therefore, further studies are needed to analyze the role of these factors.
ADSC-CM effect on the density of collagen in vivo has not been reported yet. Collagen densities in the four groups were all increased in day 28, compared to day 14. This fact showed that collagen has not entered remodeling phase yet. As the imbalance of collagen synthesis and reabsorption at the stage of remodeling can cause pathological scar.6,22 A longer study in terms of collagen densities is recommended.
In vivo and in vitro process of wound healing are different. In vivo wound healing occurs in a very complex process.23 Therefore, more studies are needed to analyze the role of ADSC-CM in wound healing, as ADSC-CM is a cocktail of various cytokines and growth factors that can affect various cellular and molecular activity of the wound healing process. We only analyzed EGF and VEGF levels, which might contribute to the healing process, but we did not address the other contents of ADCS-CM, and this is the limitation of our study.
In conclusion, though there was a clinical improvement in epithelialization of skin wound healing in Sprague Dawley rats, the improvement was not statistically significant, probably due to the single application of ADSC-CM.
Conflicts of Interest
Jeanne A. Pawitan is one of the editorial board members, but was not involved in the review or decision process of the article.
Acknowledgment
We acknowledge dr. Marcella E. Rumawas, M.S., Ph.D. for statistical analysis and LPPI Universitas Tarumanagara for financial support of the entire study.
REFERENCES
- Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203(1–3):93–8.
- Badan Penelitian dan Pengembangan Kesehatan Kementerian Kesehatan RI. Prevalensi cedera dan penyebabnya. In: Riset kesehatan dasar Riskesdas 2013. Jakarta; Kementerian Kesehatan RI:2013. p. 101. Indonesian.
- Dinh T, Braunagel S, Rosenblum BI. Growth factors in wound healing; the present and the future? Clin Podiatr Med Surg. 2015;32(1):109–19.
- Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impared wound healing. Cytokine. 2015;71(2):409–12.
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanism. J Int Med Res. 2009;37(5):1528–42.
- Harper D, Young A, Mc Naught C-E. The physiology of wound healing. Surgery. 2014;32:445–50.
- Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849.
- Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24(2):136–43.
- Walter MNM, Wright KT, Fuller HS, MacNeil S, Johnson WEB. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res. 2010;316:1271–81.
- Kim J, Lee JH, Yeo SM, Chung HM, Chae JI. Stem cell recruitment factors secreted from cord blood-derived stem cell that are not secreted from mature endothelial cells enhance wound healing. In Vitro Cell Dev Biol. 2014;50(2):146–54.
- Greaves NS, Ashcorft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci. 2013;72(3):206–17.
- Chen D, Hao H, Fu X, Han W. Insight into reepithelialization: how do mesenchymal stem cells perform? Stem Cells Int. 2016;2016:6120173.
- Bellavia G, Fasanaro P, Melchionna R, Capogrossi MC, Napolitano M. Transcriptional control of skin reepithelialization. J Dermatol Sci. 2014;73(1):3–9.
- Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70(12):2059–81.
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304(1):274–86.
- Kwon SH, Bhang SH, Jang HK, Rhim T, Kim BS. Conditioned medium of adipose-derived stromal cell culture in three-dimensional bioreactors for enhanced wound healing. J Surg Res. 2015;194(1):8–17.
- Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22(4):862–72.
- She T, Hu D, Zhang J, Zhang W, Liu J, Chen G, et al. Cytobiological effect of adipose-derived stem cells treated with insulin on HaCat cells. Zhonqquo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(6):727–31.
- Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, et al. Hair growth stimulation by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31(1):27–34.
- Kryger Z, Zhang F, Dogan T, Cheng C, Lineaweaver WC, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: exaination of various routes of adminitration. Br J Plast Surg. 2000;53(3):234–9.
- Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–21.
- Hu L, Zhao J, Liu J, Gong N, Chen L. Effects of adipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp Ther Med. 2013;5:701–6.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id