
Section Abstract Introduction Methods Results Discussion Conflict Of Interest Acknowledgment References
Basic Medical Research
The preventive effect of Mangifera foetida L. leaf extract administered simultaneously to excess iron on markers of iron overload in Spraque-Dawley rats
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i4.1829 Med J Indones. 2017;26:246–52
Received: February 8, 2017
Accepted: December 30, 2017
Author affiliation:
1 Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
2 Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 German-Indonesian Medical Association (GIMA, DIGM e.V.)
Corresponding author:
Ari Estuningtyas
E-mail: ari_handoyo@yahoo.com
Background
Recently, there is no agent available for the prevention of iron overload (IO) in thalassemia patients. Previous studies showed that Mangifera foetida L. leaf extracts reduced the levels of iron in IO in vitro and in vivo models. The present study aimed to determine the efficacy of Mangifera foetida L. leaf extract in the prevention of IO induced in rats.
Methods
Thirty male Sprague-Dawley rats were divided into 5 groups: control (untreated), IO, 3 treatment groups with leaf extract equivalent to 50, 100, and 200 mg of mangiferin per kg BW. Fe-dextran (15 mg) was administered intraperitoneally twice a week for 4 weeks to all groups except control (IO, DSM50, DSM100, and DSM200). Urine and blood samples were taken before and after treatments. After 4 weeks of treatment, rats were terminated, and samples of spleen, liver, and heart were taken. Ferritin and mangiferin levels and SOD activities were determined in plasma. Iron levels were measured in plasma, urine, and spleen.
Results
Experimental IO increased plasma Fe content 4.23 times and plasma ferritin levels 6.9 times vs normal. Mangifera foetida L. leaf extract DSM50 resulted in the highest blood levels of 212 ng mangiferin per mL and moderately, although not significant, prevented increased plasma ferritin levels and IO in organs and protected against oxidative stress.
Conclusion
Aqueous Mangifera foetida L. leaf extract may be useful to prevent IO and oxidative stress in thalassemia patients.
Keywords
Fe level, iron overload, Mangifera foetida L. leaf extract, mangiferin, SOD activity
Iron overload (IO) is a serious chronic condition that develops when the body absorbs too much iron over years, and then excess iron is accumulated in organ tissues.1 In thalassemia, iron overload can cause progressive organ injury before clinical symptoms develop.1 Thalassemia is widely spread over the world covering the Mediterranean, Africa, Middle East, Burma, South East Asia, and Southern China.2 In Indonesia, the prevalence of thalassemia is 0.1% with the highest numbers in Nanggroe Aceh Darussalam, South Sumatera, and Riau island.3
Thalassemia is an inherited disorder caused by mutations and/or deletions that decrease the synthesis of α- or β-globin chains. Impaired hemoglobin synthesis induces increased iron absorption from the gastrointestinal (GI) tract. In β-thalassemia major, severe anemia with massive erythroid hyperplasia in the bone marrow and heavy cachexia can occur.4
The supportive management of thalassemia includes blood transfusions to maintain hemoglobin levels of at least 9 to 10 g per deciliter (dL). These sporadic transfusions in thalassemia intermedia and regular transfusions in thalassemia major patients ameliorate anemia, improve the oxygen uptake into tissues, and reduce hematopoietic stimuli that can cause hepatosplenomegaly and bone deformities.5
Increased iron absorption from the GI tract of thalassemia patients and receiving (regular) blood transfusions leads to iron overload, frees iron in blood circulation, and accumulates iron in the visceral organs (e.g., liver, endocrine glands) and the heart. Excess iron in the systemic circulation can saturate transferrin (iron-binding protein in plasma). As a result, free iron will bind to other compounds with low molecular weight, e.g., citrate. Non transferrin-bound iron (NTBI) is easily absorbed by certain cells, including hepatocytes and cardiomyocytes. NTBI is also very sensitive to oxygen directing Fe(II) to the Fenton reaction which will generate superoxide anion radical and hydrogen peroxide.1,2 Iron overload in myocardium and the iron-mediated generation of free radicals are associated with cardiovascular and other diseases and may eventually cause myocardial failure.6
Iron chelating agents were introduced in 1960 for the treatment of iron overload. The use of chelating agents aims to bind iron and prevent the formation of iron-mediated free radicals. Presently, deferoxamine, deferasirox, and deferiprone are used for iron chelating therapy, but all of them can cause severe unwanted side effects. Deferoxamine as the main standard drug has additional drawbacks such as lack of intestinal absorption and thus the inconvenience of parenteral application. Deferiprone and deferasirox can be administered orally, but they are too expensive for regular administration to thalassemia patients in Indonesia. Hence, it is necessary to search for novel chelating agents from domestic plants.7
One of the alternatives as chelating agent is mangiferin, which is contained Mangifera species, e.g., Mangifera foetida L. and some other medicinal plants. Many studies indicate that mangiferin exerts a wide pharmacological range, e.g., as antidiabetic, anti-HIV, anticancer, immunomodulatory, antioxidant, and iron chelating agent.8–11 Its ironchelating and free radical scavenging properties can prevent the early stages in the iron-induced formation of hydroxyl radicals.12
In rats with experimentally established iron overload, the therapeutic application of mangiferin and Mangifera foetida leaf extract reduced high plasma iron concentrations.13 However, the prevention of iron overload in vivo has not yet been investigated. Therefore, the present work aimed to study the preventive effect of Mangifera foetida leaf extract towards the iron overload in Spraque-Dawley rats induced by administration of excess iron.
METHODS
Materials
Spraque-Dawley strain rats were purchased from the Indonesia National Agency of Drug and Food Control. Mangifera foetida L leaf powder was purchased from the Research Institute for Spices and Medicinal Plants (Bogor, Indonesia). Iron dextran (Fahrenheit, Indonesia), epinephrine (Sigma Aldrich, US), NaHCO3 (Merck, Germany), NaEDTA (Merck, Germany), rat ferritin enzimlinked immunosorbent assay (ELISA) kit (Genway, US), TLC plate (Merck, Germany), mangiferin reference (Sigma Aldrich, US) were obtained at the highest purity available.
Mangifera foetida L. leaf extract
Mangifera foetida L. leaves were obtained from LIPI Cibinong in the form of analyzed dry powder. Dried powder was extracted using infundation at a temperature of 70°C for 15 minutes. This method refered to the research previously performed by Wahyuni.13
Mangifera foetida leaf extracts were prepared by infusion of 1 part of dried leaf powder in 5 parts of water at 70°C for 15 minutes and processed to obtain a mangiferin-containing extract. Each infusion was carried out in triplicate. The solvent was evaporated with vacuum evaporator at 60°C and 74 mBar pressure to obtain a viscous ‘thick extract’.
Study design
This experimental study was conducted in Animal House and Pharmacology Laboratory from July 2014 until November 2014. Thirty male Spraque- Dawley rats were divided into five groups of 6 rats, each (normal, iron overload (IO), DSM50, DSM100, DSM200). The normal control group received no treatment. The animals in the iron overload group were administered iron dextran 15 mg intraperitoneally, twice a week for 4 weeks. Extract groups (DSM) were administered Mangifera foetida leaf extract equivalent to mangiferin 50, 100 or 200 mg/kg body weight (BW) daily for 4 weeks and iron dextran 15 mg intraperitoneally, twice a week for 4 weeks. After 4 weeks, all rats were killed. The blood of each rat was drawn. Moreover, spleen, heart, and liver were excised.14,16 This study was approved by the Health Research Ethics Committee Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital No 205/H2.F1/ETIK/2013.
Thin layer chromatography
Thin layer chromatography (TLC) was used to identify mangiferin content in the extract. TLC was performed using methanol-acetic acidwater (9:0.5:0.5) as mobile phase.13 Mangiferin plasma concentration was analyzed using high performance liquid chromatography (HPLC) with a C18 column and PDA detector. The mobile phase was composed of methanol and 0.5% aqueous formic acid (30:70). The mobile phase was filtered through 0.22 μm membrane and degassed by ultrasound. The isocratic elution run time was 10 min at a flow rate of 1.0 mL per min.14
Further determinations
The measurements of iron were performed using atomic absorption spectroscopy (AAS). Samples were prepared by destruction of plasma, urine, and spleen sample with 1.0 mL nitric acid. Subsequently, the solution was heated on a hot plate until brown vapor disappeared. Furthermore, the solution was added to 5 mL of distilled water, and the iron content was measured.15
Superoxide dismutase (SOD) activity assay was carried out in accordance with Fridovic methods.16 Ferritin plasma concentration was analyzed using Rat Ferritin-GWB-76152C ELISA kit Genway.
RESULTS
Mangiferin levels
Mangiferin was identified in the extract by thin layer chromatography (TLC). TLC of the ethanol fraction of the Mangifera foetida L. extract showed two spots with Rf 0.84 and 0.43 (Figure 1). Similar Rf values of the extract spot with the mangiferin standard Rf indicated mangiferin in the extract. Besides mangiferin, the extract also contained other compounds marked by other extract spots (Figure 1, lanes 2), i.e., alkaloids, flavonoids, saponins, and triterpenoid.
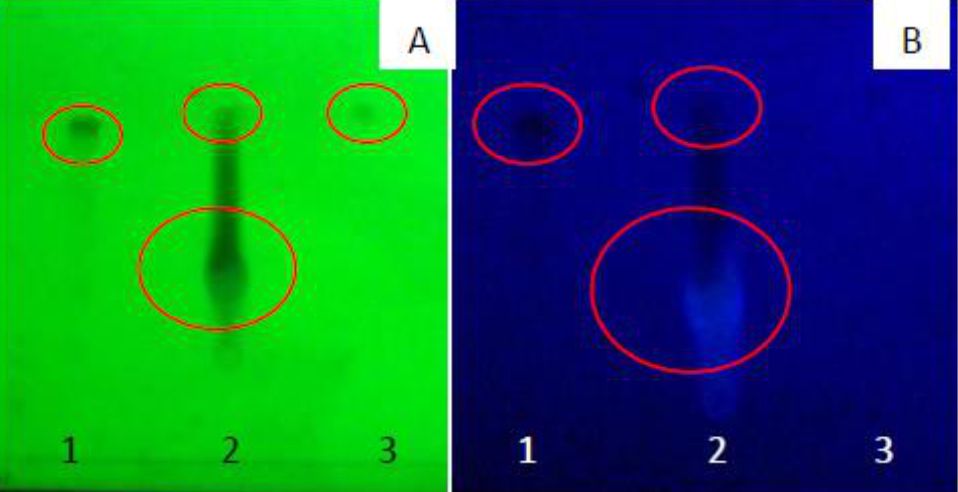
Figure 1. TLC of Mangifera foetida L. leaf extract detected at λ 254 nm (A) and λ 366 nm (B). Lane 1, mangiferin standard; lane 2, ethanol fraction; lane 3, hexane fraction
Furthermore, mangiferin was also proven by measuring mangiferin levels in the plasma of rats treated with Mangifera foetida leaf extract at week 4 of the experiment: DSM50 group 212 ng/mL; DSM100 group 116 ng/mL; DSM200 group 145 ng/mL (Figure 2). The peak obtained from Mangifera foetida leaf extract had the same retention time as the standard mangiferin indicating identical compounds.

Figure 2. Mangiferin levels in plasma after 4 weeks of treatment with Mangifera foetida L. leaf extract
Plasma iron and ferritin
The experimental induction of IO in rats successfully increased plasma Fe content by 4.23 times and plasma ferritin levels by 6.9 times compared with the normal group (Figure 3 and 4).
The concomitant administration of Mangifera foetida L. leaf extract for 4 weeks lowered iron plasma levels in the DSM100 group and ferritin levels in the DSM50 group although statistically it was not significant (Figure 3 and 4).
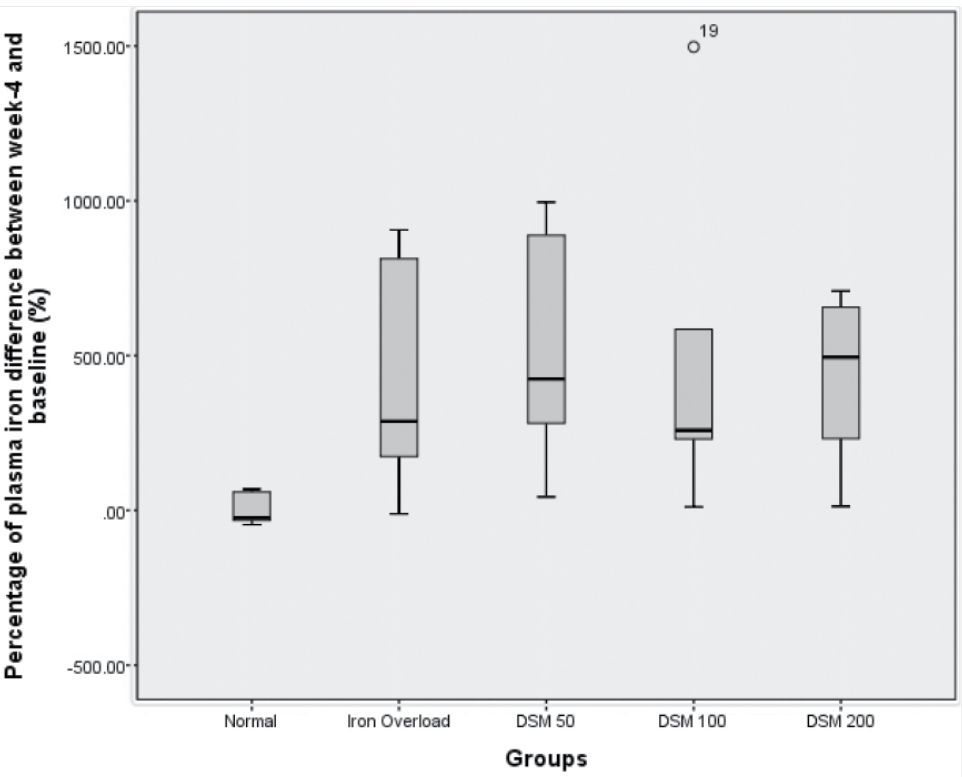
Figure 3. Percentage of plasma iron showing the differences between baseline and 4 weeks of iron administration
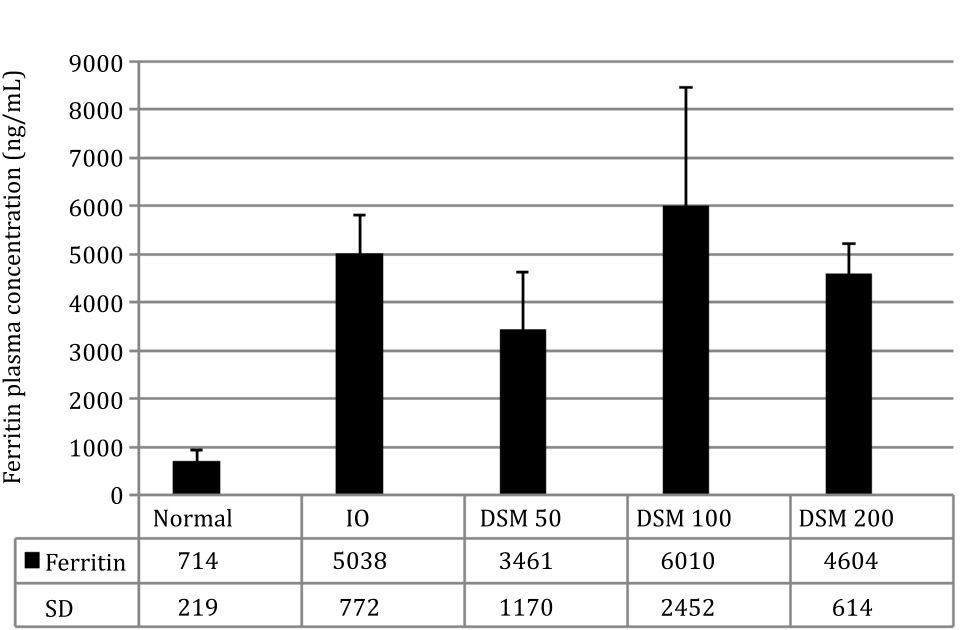
Figure 4. Ferritin plasma concentration
Organ iron content
After four weeks of experimental iron overload and administration of Mangifera foetida L. leaf extracts, the organs (heart, liver, and spleen) in the iron overloaded groups had darker color compared to the normal group. The color change Figure 2. Mangiferin levels in plasma after 4 weeks of treatment with Mangifera foetida L. leaf extract Figure 3. Percentage of plasma iron showing the differences between baseline and 4 weeks of iron administration Figure 4. Ferritin plasma concentration indicates the elevated levels of iron in the respective groups.
The spleen weight showed differences between the IO (1.26 g) and DSM50 (0.92 g) and DSM200 (0.83 g) although statistically it was not significant, indicating an influence of mangiferin on iron distribution into the spleen. Measurement of the total iron content in the spleen showed that DSM50 group had about 10% and DSM200 12% less total iron than the IO group (Figure 5).
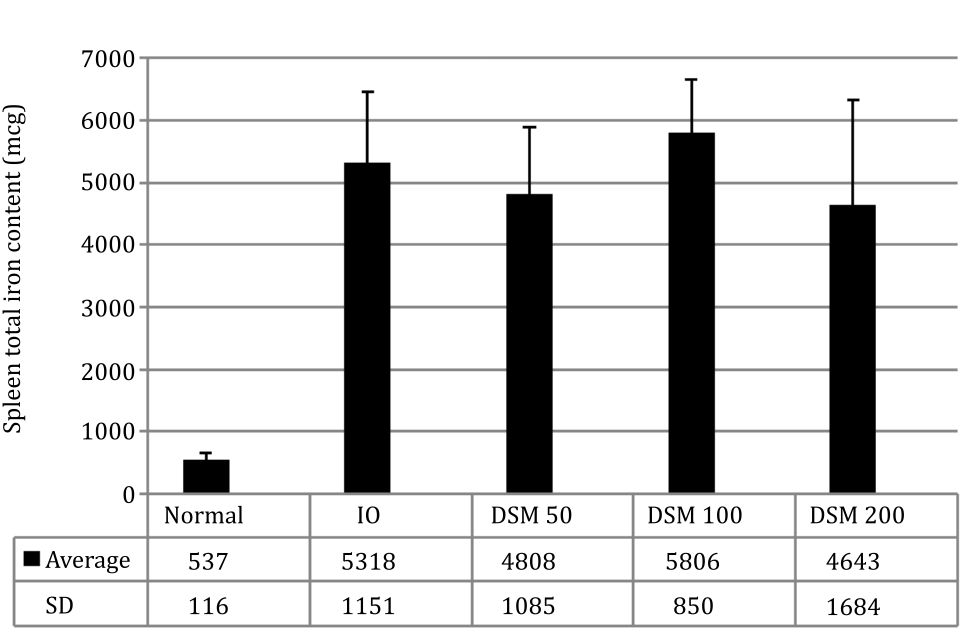
Figure 5. Spleen total iron content
SOD activity
After 4 weeks, SOD activity in plasma decreased along with the induction of iron overload (Figure 6). The administration of Mangifera foetida L. leaf extract equivalent to the lowest dose of 50 mg mangiferin (DSM 50) prevented this decrease in SOD activity, compared with the IO group. With higher doses of mangiferin, the protective effect got lost: in the DSM100 group, there was almost no protection vs. IO and in the DSM 200 group, SOD activity was even lower than in IO alone (Figure 6).
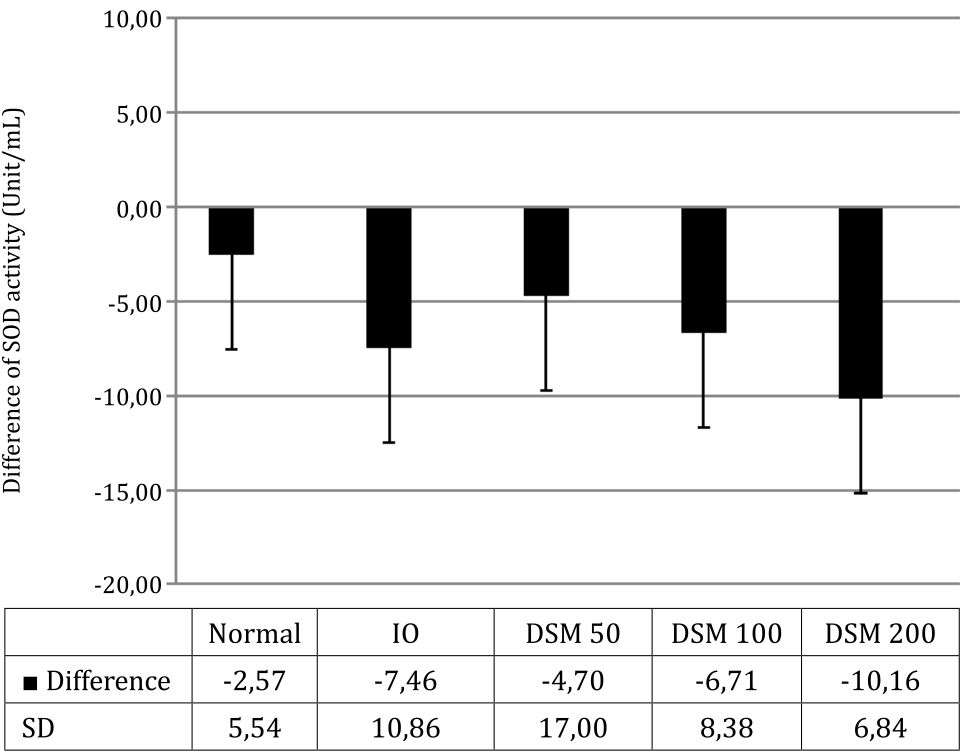
Figure 6. SOD activity, difference from baseline after 4 weeks of treatment
DISCUSSION
Iron-overload is a common condition in thalassemia patients due to increased absorption from the gastrointestinal tract and additional blood transfusions.1,5 High levels of iron concentration in thalassemia patients lead to increased free radical formation mediated by NTBI. The increase in free radicals is associated with final cardiovascular complications as a major cause of deaths in thalassemia patients.7
Mangiferin as a lead compound in Mangifera foetida L. leaf extracts has been known for its antioxidant activity. There are two antioxidant mechanisms of mangiferin against free radicals induced by iron; firstly, it prevents free radical generation by chelating iron and secondly, it scavenges free radicals.17
Water extract from leaves was used in this study because the glucoside of native mangiferin has better solubility in water than in ethanol. According to Kim et al,18 mangiferin has the highest solubility in methanol followed by water and ethanol. We preferred water as extraction medium because it is less toxic than methanol, and aqueous extract is most easily made by the community.
Several studies have been conducted to assess the potential of Mangifera foetida L. leaf extract towards iron-overload. An ex-vivo study conducted by Pohan et al.17 showed that mangiferin from Mangifera foetida L. leaf extract can bind to ferritin in blood plasma of patients with thalassemia. In another study, administration of Mangifera foetida L. leaf extract was equivalent to 75 mg/kg BW of mangiferin in experimentally iron-overloaded rats reduced iron plasma levels by 51% after one week of therapy and increased the elimination of 90% of Fe in urine.13 The study showed that Mangifera foetida L. leaf extract had the pharmacological potential of reducing iron levels in iron-overloaded conditions. Therefore, the aim of the present work was to further document the ability of Mangifera foetida L. leaf extract in preventing elevation of iron levels in rats administered with excess iron.
The highest blood levels were obtained with DSM50. Increasing doses of the extract in the DSM100 and DSM200 groups did not lead to doseresponse relationship in blood levels indicating non-linear pharmacokinetics of mangiferin.
Mangiferin has poor solubility and permeability in the gastrointestinal (GI) tract, with only 1.2% bioavailability.19 Increasing doses of the extract are suspected of causing conglomerations, which make mangiferin absorption from the GI tract more difficult. In several studies, administration of more than 40 mg mangiferin resulted in non-linear pharmacokinetics of its absorption process.19 From these results, only the administration of the extract equivalent to 50 mg of mangiferin per kg BW can be accounted for pharmacological effects.
In this study, the administration of Mangifera foetida L. leaf extract started when plasma iron conditions were still normal. Under normal conditions, almost all iron circulating in the blood binds to transferrin. Through the strong iron-transferrin, binding mangiferin cannot bind free iron and remove it from the body. Hence, the preventive effect will not be very strong and only moderate. Possibly, no significant pharmacological effects might be expected. That does not mean that mangiferin and the applied extract are ineffective because the iron-binding and iron-removing effect may increase with the increasing iron overload.
Plasma ferritin concentration is an indicator of iron-overload in humans and in this condition, therapy with chelating agents is initiated when ferritin levels are above 1,000 ng/mL or 10 times the normal ferritin concentration. At high ferritin concentrations above 3,000 ng/mL or 30 times the normal male ferritin levels, treatment with chelating agents decreased iron more effectively than at lower ferritin concentrations.20 This demonstrates the relationship between the plasma iron concentration and the effectiveness of chelating agents in binding it. In this study, plasma ferritin concentration in the IO group was 5,038 ng/mL or 7 times the concentration of the normal group. Thus, the concentration of ferritin in this study was intentionally lower than the concentration of ferritin in thalassemia patients when iron chelation therapy is started. Low plasma levels of iron and ferritin appear to be the cause of the relatively weak capability of Mangifera foetida leaf extracts to prevent the increase of iron in the body or to remove it through the urine (not shown).
The study design of Wahyuni13 differed from this study. The former study examined the therapeutic effects of Mangifera foetida L. leaf extract towards experimentally established IO, whereas this study investigated whether this extract had also an effect in preventing experimentally induced IO in rats. The effect of Mangifera foetida L. leaf extract was much more expressed in the former13 than in this preventive study design. In the condition of iron overload,13 plasma transferrin was fully saturated, and high NTBI was formed that was easily bound by mangiferin. Our result raised an assumption that mangiferin from the extract was bound to plasma transferrin or ferritin and prevented the iron release from these proteins, thus diminishing the formation of NTBI. In a previous ex-vivo-study with thalassemia patients, Mangifera foetida leaf extract formed a complex with ferritin in serum.21 Another assumption is that Mangifera foetida L. leaf extract increased the expression of iron binding proteins like transferrin and ferritin and inhibited the generation of NTBI via this mechanism.
SOD is an enzyme that converts superoxide anion radical into hydrogen peroxide in the body. Hydrogen peroxide is formed and then is converted into water and oxygen by catalase. Thus, SOD provides protection against superoxide free radicals by transforming them into compounds that are not toxic to the body. When the body is moderately exposed to free radicals, gene transcription of endogenous antioxidants is induced which leads to increased antioxidant activities that can detoxify free radicals.22
In this study, SOD activity in plasma decreased by the induction of iron overload. The ability of SOD in protecting tissues against free radicals is greatly affected by its concentration and the severity of the radical attack. SOD dose response curve to the protection capability is bell-shaped. In early stages of moderate oxidative stress, increased SOD expression and activity improved the protection against free radicals. Further increase of SOD concentration lowered the protection against free radicals. In studies conducted by Pardo-Andreu et al12 and Wahyuni,13 plasma SOD activity decreased with free iron confirming the results of this study.
Under massive experimental and pathological oxidative stress in skin, SOD activity decreased.23 It was proposed that the increase in SOD activity develops superoxide radical dismutation to hydrogen peroxide, which in turn accumulates and attacks biomolecules, thus reducing the allover protective effects of SOD.24
DSM50 showed a protective effect in this study, but this protective effect decreased with increasing doses of the extract. In the DSM200 group, there was significant decrease of the antioxidant activity indicating – under linear pharmacokinetic conditions - DSM overdose in this group leading to prooxidant activity. Mangifera foetida L. leaf extract contains mangiferin, flavonoids, and terpenoids. Mangiferin itself is known to have antioxidant and prooxidant activities. The mechanism of antioxidant mangiferin is iron binding, therefore inhibiting the formation of hydroxyl radicals and free radicals by scavenging with its phenolic catechol group. When mangiferin captures free radicals, its catechol group is oxidized transiently forming a semiquinone radical, which is potentially toxic to biomolecules.25 This raises the suspicion that increasing doses of mangiferin may cause an increase in semiquinone radicals with increasing prooxidant effects rather than the antioxidant effects of lower doses. Moreover, other compounds in the extract such as flavonoids and terpenes may affect SOD activity in our experiments.
However, since higher doses of the applied extract created lower rather than higher blood levels of mangiferin, this non-linear pharmacokinetic effect should be accounted for the lower antioxidant effect of DSM200.
In conclusion, Mangifera foetida leaf extract equivalent to 50 mg mangiferin per kg BW exerts moderately preventive effect on the parameters of experimentally induced iron overload in rats. Higher doses of mangiferin are not more effective because of non-linear pharmacokinetics. Thalassemia patients can be advised to use Mangifera leaves and fruit peels for preparing an aqueous infuse for daily drinks. Future research should better clarify the activities of Mangifera foetida L. leaf extract in the prevention of iron-overload. Some changes in study design, such as longer iron dextran induction period and/or extract administration can be expected to give more information about the capability of extract components, especially mangiferin to interact with iron-binding proteins, also using computersimulated molecular docking.
Conflicts of Interest
Melva Louisa and Hans-Joachim Freisleben are the editorial board members, but were not involved in the review or decision process of the article.
Acknowledgment
This study was funded by Direktorat Riset dan Pengabdian Masyarakat Universitas Indonesia, 2013.
REFERENCES
- Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366(4):348–59.
- Olivieri NF. The β-Thalassemias. N Engl J Med. 1999;341(2):99–109.
- Depkes. Riset kesehatan dasar 2007. Jakarta: Badan Penelitian dan Pengembangan Kesehatan; 2008. Indonesian.
- Aster JC. Hematopoietic and lymphoid systems. In: Kumar V, Abbas AK, Aster JC, eds. Robins Basic Pathology. 9th ed. Philadelphia: Elsevier. 2013:413–6.
- Rund D, Rachmilewitz E. β-Thalassemia. N Engl J Med. 2005;353(11):1135–46.
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53(2):75–100.
- Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med. 2011;364(2):146–56.
- Ichiki H, Miura T, Kubo M, Ishihara E, Komatsu Y, Tanigawa K, et al. New antidiabetic compounds mangiferin and its glucoside. Biol Pharm Bull. 1998;21(12):1389–90.
- Wang RR, Gao YD, Ma CH, Zhang XJ, Huang GC, Huang JF, et al. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules. 2011;16(5):4264–77.
- Yoshimi N, Matsunaga K, Katayama M, Yamada Y, Kuno T, Qiao Z, et al. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001;163(2):163–70.
- Leiro J, Arranz JA, Yáñez M, Ubeira FM, Sanmartın ML, Orallo F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol. 2004;4:763–78.
- Pardo-Andreu GL, Barrios MF, Curti C, Hernández I, Merino N, Lemus Y, et al. Protective effects of Mangifera indica L extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacol Res. 2008;57(1):79–86.
- Wahyuni T. Pengaruh mangiferin dan ekstrak air daun Mangifera foetida L sebagai zat pengkelat besi dan antioksidan secara in vivo pada tikus Sprague Dawley. Tesis Fakultas Kedokteran Universitas Indonesia. 2013. Indonesian.
- Hou S, Wang F, Li Y, Li Y, Wang M, Sun D, et al. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012;132(1):289–94.
- Papanastasiou DA, Vayenas D V, Vassilopoulos A, Repanti M. Animal and in vitro models in human disease concentration of iron and distribution of iron and transferrin after experimental iron overload in rat tissues in vivo: study of the liver, the spleen, the central nervous system and other organs. Pathol Res Pract. 2000;196(1):47–54.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–517.
- Pardo-Andreu GL, Delgado R, Núñez-Sellés AJ, Vercesi AE, Gilberto L. Dual mechanism of mangiferin protection against iron-induced damage to 2-deoxyribose and ascorbate oxidation. Pharmacol Res. 2006;53(3):253–60.
- Kim W-J, Veriansyah B, Lee Y-W, Kim J, Kim J-D. Extraction of mangiferin from Mahkota Dewa (Phaleria macrocarpa) using subcritical water. J Ind Eng Chem. 2010;16(3):425–30.
- Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. 2014;93:54–61.
- Porter JB. Practical management of iron overload. Br J Haematol. 2001;115(2):239–52.
- Pohan APN, Purwaningsih EH, Dwijayanti A. Efek kelasi ekstrak etanol daun Mangifera foetida pada feritin serum penderita talasemia di RS Cipto Mangunkusumo, Tahun 2012. eJKI. 2013;1(1):45–52. Indonesian.
- Li Y. Antioxidants in biology and medicine. New York: Nova Science Publishers; 2011.
- Fuchs J, Freisleben H-J, Packer L. Antioxidants in the skin. In: Mukhtar H, ed. Pharmacology of the Skin. Boca Raton: CRC Press; 1991. p. 249–67.
- McCord JM. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response. 2008;6(3):223–38.
- Pardo-Andreu GL, Cavalheiro RA, Dorta DJ, Naal Z, Vercesi E, Curti C. Fe (III) shifts the mitochondria permeability transition-eliciting capacity of mangiferin to protection of organelle. J Pharmacol Exp Ther. 2007;320(2):646–53.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id