
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Brief Communication
The profile of codon 200 β-tubulin gene of Ascaris lumbricoides L. and Trichuris trichiura L. from infected people in Nangapanda Sub-district, East Nusa Tenggara
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v27i4.1935 Med J Indones. 2018;27:304–9
Received: March 30, 2017
Accepted: September 12, 2018
Author affiliation:
1 Department of Parasitology, Faculty of Medicine, Universitas Trisakti, Jakarta, Indonesia
2 Master of Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
3 Department of Parasitology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Corresponding author:
Yenny Djuardi
E-mail: yenny_djuardi@yahoo.com
Background
The mass administration of anthelmintic such as albendazole is one of the strategies for eliminating soil-transmitted helminth (STH) infection. The widespread and long-term use of anthelmintics can cause resistance. The research on animals shows that factor that the single-nucleotide polymorphism (SNP) codon 200 β-tubulin gene of the worms is one of the factors that can cause the decreased efficacy of anthelmintics. This study aimed to determine the bases of codon 200 in A. lumbricoides and T. trichiura, which infect the people in Nangapanda, East Nusa Tenggara.
Methods
The worm samples were obtained from the intestinal helminth-infected patients from Nangapanda Sub-district. The DNA from the worm tissues were isolated, amplificated by polymerase chain reaction (PCR), and sequenced. The sequencing results were aligned to the reference sequence to obtain the codon bases in the 200 β-tubulin gene.
Results
TTC constitute the codon bases in the 200 β-tubulin gene found in two A. lumbricoides and one T. trichiura.
Conclusion
The SNP codon 200 β-tubulin gene was absent in A. lumbricoides or T. trichiura worms that were examined in this study.
Keywords
β-tubulin gene, Ascaris lumbricoides, single nucleotide polymorphism (SNP) codon 200, Trichuris trichiura
Soil-transmitted helminth (STH) is one of the parasites that cause the most common infection in the world. More than 1 billion people have been infected, with 300 million showing clinical symptoms of severe infection by more than one species of STH.1–3 The mass anthelmintic treatment is one of the strategies for STH elimination.4 The World Health Organization (WHO) recommends the use of mebendazole or albendazole in the elimination program. The repeated and regular elimination program of those at risk ensures the low level of infection.5 However, the long-term use of anthelmintic drugs can lead to resistance or at least decrease of the drug efficacy to combat the STH infection.5,6 A meta-analysis study in 2008 showed that the single-dose administration of 400 mg albendazole produces 88% cure rate against A. lumbricoides and 28% against T. trichiura.7 Although the decline in the efficacy of albendazole in humans have been reported, the resistance to albendazole has not been confirmed.8
The albendazole acts against helminths through the binding of β-tubulin, one of the proteins that form microtubule.9,10 The microtubule is an important organelle for motility, cell division, and the secretion of nucleated cells in all living things.11,12 The binding of β-tubulin in STH will disrupt the microtubule polymerization, hampering the entire process of worm energy management and eventually resulting in the death of the worm or its removal from the body of the host.3,9
Several factors could contribute to the decreased efficacy of benzimidazole, i.e. host, parasite, and drug. One of the factors involving parasites is single-nucleotide polymorphisms (SNPs), for example, the substitution of phenylalanine to tyrosine in codon 200 β-tubulin gene (Phe200Try).1,13,14 Several animal studies discovered a polymorphism in β-tubulin gene isotype 1 in the parasitic nematode Haemonchus contortus; this polymorphism leads to albendazole resistant in Kuningan, Yogyakarta, and Bogor.13,14 This SNP was also discovered in the albendazoleresistant Trichostrongylus worms.15
The human studies in Haiti and Kenya identified the SNP codon 200 in T. trichiura (homozygote and heterozygote TAC) but not in A. lumbricoides (all homozygote TTC).1 Whether the parasitic worms A. lumbricoides and T. trichiura infecting the population in Indonesia feature an SNP profile similar to that found in Haiti and Kenya remains unknown. A study on a population in Nangapanda Sub-district, East Nusa Tenggara showed that after 7 rounds of three-month mass treatment with a single dose of albendazole, the percentage of T. trichiura infection decreased from 27.8% to 17.7% whereas A. lumbricoides infection decreased from 33.2% to 9.7%.16 Based on the differences in the decreased percentage in both species of STH, this study aimed to determine the profile of codon 200 β-tubulin gene in A. lumbricoides and T. trichiura infecting residents in Nangapanda Sub-district, Ende, East Nusa Tenggara.
METHODS
Sample collection
The worm samples were obtained from the school children in Nangapanda Sub-district, Ende, East Nusa Tenggara after the administration of albendazole treatment in accordance with the national program. The worm samples consisted of four A. lumbricoides worms (three females and one male) and one female T. trichiura. Each of the A. lumbricoides worms was obtained separately from four school-age children who had been given a single dose of 400 mg albendazole. On the other hand, the T. trichiura worm was obtained from school-aged children who had been given pyrantel pamoate. These worms were stored at a temperature of -20 °C before analysis. This study was approved by the Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia (No. 194/PT02.FK/Etik/2006).
Isolation of DNA worms
Prior to the isolation of A. lumbricoides worm, 0.25 g of worm tissue (posterior part) was weighed, cut into small pieces, and placed in a cryotube. The T. trichiura worm was inserted as a whole into another cryotube. Next, liquid nitrogen was added gradually to the sample while being crushed repeatedly with a glass rod. The procedure of DNA isolation was performed according to the DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany).17 A total of 20 μl proteinase K was added to the samples, which were then vortexed until homogeneity. The samples were then incubated at 56°C for 1 h, vortexed, and spun down. Next, 200 μl of AL buffer was added followed by 200 ml of ethanol (98%–100%), with each adding step followed by vortexing and spinning down. All the solutions were then placed in the DNeasy spin column placed in a 2 ml tube and were centrifuged with a speed of 8,000 rpm for 1 min. The filtrate was then discarded, and 500 μl of AW1 buffer was added. Afterward, the tubes were centrifuged at a speed of 8,000 rpm for 1 min. The filtrate was discarded, and 500 μl of AW2 buffer was added. Then, the tubes were centrifuged at a speed of 14,000 rpm for 3 min. The filtrate was removed, and the tubes were centrifuged again without adding anything at the speed of 14,000 rpm for 1 min. The spin columns were then transferred into two new tubes, and 100 μl buffer AE was added. The tubes were incubated at room temperature and were centrifuged at a speed of 8,000 rpm for 1 min. The DNA concentration in the elution was measured using NanoDrop (Implen, Germany). The DNA samples were stored at -20 °C before use.
Polymerase chain reaction (PCR) and gel electrophoresis
A total of 25 μl KAPA Taq HotStart Extra Readymix (Kapa Biosystems, MA., USA) was placed in a PCR tube and was added with 2.5 μl forward primer, 2.5 μl reverse primer for A. lumbricoides, 15 μl RNA-free water, and 5 μl samples. The negative control contained RNA-free water. The tube was placed in the Rotor-gene PCR machine (Qiagen, Hilden, Germany), and PCR was conducted at an initial denaturation temperature of 95 °C for 3 min, followed by denaturation temperature of 95 °C for 30 s, annealing temperature of 54.3 °C for 30 s, extension temperature of 72 °C for 1 min (denaturation temperature to extension temperature was then repeated 35 times), and a final extension temperature of 72 °C for 1 min (Table 1).
Table 1. Primers used for PCR1

The PCR results were visualized using electrophoresis. The target band was the β-tubulin amplicon of A. lumbricoides with a length of 158 bp, whereas that of T. trichiura exhibited a length of 163 bp.3 A total of 4 μl of PCR products (samples and negative controls) and 4 μl of 100 bp ladder were mixed with 2 μl loading dye and 4 μl SYBR® green (1:1000). The mixtures were then added to the wells with 1.5% agarose gel. The electrophoresis machine was run with 118 mA, 85 V, 10 W for 45 min. The results were read in the FireReader V4 Gel Documentation Machine (UVItec, UK).
Sequencing
PCR samples were then sent to 1st Base to be purified and sequenced using the Sanger method. The sequences were entered into the Basic Local Alignment Search Tool (BLAST) program to determine the similarity between the sample sequences and reference sequences in GenBank. BLAST program can be found at http:// blast.ncbi.nlm.nih.gov/Blast.cgi.
Alignment of sequence data
The alignment was done by using BioEdit Sequence Alignment Editor version 5.0.9 with EU814697.1 as a reference for A. lumbricoides and KF410623.1 for T. trichiura.
RESULTS
DNA Isolation
The DNA concentration obtained from the worm tissues ranged from 109 ng/ml to 233 ng/ml for A. lumbricoides and 9.65 ng/ml for T. trichiura. From these samples, the isolation of A. lumbricoides DNA from sample Y2 yielded the highest concentration (233 ng/μl) with the highest purity as shown by A260/280 of 1.769 (Table 2). The sample of T. trichiura showed a concentration of 9.65 ng/μl with a ratio A260/280 of 1.752 (Table 2).
Table 2. DNA isolation from worm’s tissue
PCR
From the four DNA samples of A. lumbricoides, two samples were successfully amplified, Y1 and Y2, along with the sample of T. trichiura (Tt). Bands Y1 and Y2 measured about 200 bp in length, whereas Tt featured a length of about 163 bp (Figure 1). As the PCR of the remaining two samples of A. lumbricoides tissue (Y3 and Y4) failed to show any band, they were excluded from sequencing.
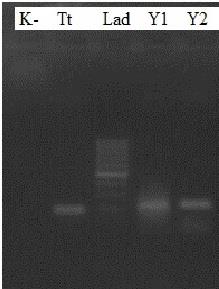
Figure 1. Electrophoresis of DNA samples from T. trichiura and A. lumbricoides adult worms. (K)=negative control; (Tt)=DNA samples of T. trichiura adult worm, (Y1 and Y2)=DNA samples of A. lumbricoides adult worm
Sequencing
Figures 2 and 3 show the alignment between A. lumbricoides reference and sequences Y1 and Y2. Both codon 200 from Y1 and Y2 were similar to the reference EU8146873, that is TTC. The band length of Y1 spanned 162 bases, whereas Y2 comprised 160 bases.

Figure 2. The alignment between A. lumbricoides reference (above) and sequences Y1

Figure 3. The alignment between A. lumbricoides reference (above) and sequences Y2

Figure 4. The alignment between T. trichiura reference (above) and Tt sequence
In the T. trichiura sample, we also found TTC in codon 200 similar to the reference AF0342193 (Figure 4). The band length of Tt amplicon was 135 bases.
DISCUSSION
The sequencing was conducted using the PCR primer obtained based on the work of Diawara et al1 (A. lumbricoides differs by 151 bases from codon 200, whereas T. trichiura differs by 64 bases). The bases of codon 200 β-tubulin gene in A. lumbricoides worms consisted of TTC; the results were consistent with the findings from a previous study.1 The codon 200 β-tubulin gene in T. trichiura also contained TTC, consistent with the results of the study conducted by Hansen et al18 but not with those of the study conducted by Diawara et al,3 who discovered the bases TAC on codon 200. In this study, A. lumbricoides and T. trichiura worms were obtained from infected people with no history of mass treatment in an endemic area in Ende.
In the study by Hansen et al,18 T. trichiura worms were obtained from people in Uganda after the first treatment with mebendazole 2x100 mg for 5 days. On the other hand, in the study by Diawara et al,3 the T. trichiura worms were obtained from school-age children who have never been treated. In Diawara et al3 study, the area where the samples were obtained was near a location with a history of mass anthelmintic treatment; thus, the people from the area who have been treated possibly relocated to locations where the samples were obtained. The difference in results between samples of T. trichiura worms from this study and Hansen et al18 with the result from Diawara et al3 may be caused by differences in the treatment history.
Three worm samples were successfully amplified, two A. lumbricoides and one T. trichiura. The failure in amplification could be caused by poor DNA quality, which can be caused by sample storage conditions, such as storing at an unstable temperature or a temperatures less than -20 °C, the addition of preservatives (formalin or alcohol), or excessive melting and freezing (freeze and thaw).19 This study found that both worms which were failed to be amplified exhibited a more crumbly consistency than the other worms. The purity of DNA concentration from A. lumbricoides that were not successfully amplified ranged from 1.2 to 1.3. These results indicated that the DNA from A. lumbricoides worms still contained contaminants, such as protein and DNA phenol.20
Study with larger samples of the population from the same area must be conducted to determine the prevalence of SNP codon 200 β-tubulin in A. lumbricoides and T. trichiura worms infecting the Nangapanda population. Studies can also be conducted on the residents of other STH endemic areas with more frequent anthelmintic treatments to determine the influence of anthelmintic administration on codon 200 SNP profile β-tubulin gene in A. lumbricoides and T. trichiura worms.
In conclusion, this research showed that the bases in codon 200 β-tubulin from A. lumbricoides and from T. trichiura comprised TTC. The worms from Nangapanda individuals included in this study contained no SNP codon 200 in their β-tubulin gene.
Conflicts of Interest
The authors affirm no conflict of interest in this study. This study is funded by Universitas Trisakti (Dewan Riset Fakultas) and Universitas Indonesia. The authors have access to the study data.
Acknowledgment
We are grateful to the participants of Nangapanda Sub-district and the Filariasis Center Team Universitas Indonesia who collected the stool and worm samples. We thank Sudirman for his assistance during the laboratory work at Universitas Indonesia.
REFERENCES
- Diawara A, Halpenny CM, Churcher TS, Mwandawiro C, Kihara J, Kaplan RM, et al. Association between response to albendazole treatment and β-tubulin genotype frequencies in soil-transmitted helminths. PLOS Negl Trop Dis. 2013;7(5):e2247.
- Vercruysse J, Albonico M, Behnke JM, Kotze AC, Prichard RK, McCarthy JS, et al. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int J Parasitol Drugs Drug Resist. 2011;1(1):14–27.
- Diawara A, Drake LJ, Suswillo RR, Kihara J, Bundy DA, Scott ME, et al. Assay to detect β-tubulin codon 200 polymorphism in Trichuris trichiura and Ascaris lumbricoides. PLOS Negl Trop Dis. 2009;3(3):e397.
- Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, Silva ND, et al. Chapter 24: Helminth infections: soiltransmitted helminth infections and schistosomiasis. Disease control priorities in developing countries (second ed). Washington (DC): World Bank; 2006. 467–83.
- World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. WHO Technical Report Series Report 912. Geneva: WHO; 2002. 33–5.
- Uneke CJ. Soil transmitted helminth infections and schistosomiasis in school age children in sub-Saharan Africa: efficacy of chemotherapeutic intervention since World Health Assembly Resolution 2001. Tanzan J Health Res. 2010;12(1):86–99.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections systematic review and meta-analysis. JAMA. 2008;299(16):1937–48.
- Prichard RK. Markers for benzimidazole resistance in human parasitic nematodes? Parasitology 2007;134(8):1087–92.
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121:S113–32.
- Wecker L, Crespo LM, Dunaway G, Faingold C, Watts S. Chapter 52: Drugs to treat parasitic infection. Brody’s human pharmacology molecular to clinical (fifth ed). Philadelphia, PA (US): Mosby and Elsevier. 2010. 621–30.
- Campbell NA, et al. Sitoskeleton adalah jejaring serat yang mengorganisasi struktur dan aktivitas dalam sel. Biologi (edisi delapan). Jakarta: Erlangga; 2010. 120–6.
- Kukuljan I. Microtubules: from classical properties to quantum effects in human cognition. Seminar class at the Faculty for Mathematics and Physics of University of Ljubljana. 2013:1–15.
- Haryuningtyas D, Beriajaya, Gray GD. Anthelmintic resistance against benzimidazole group on sheep and goat. Seminar nasional teknologi peternakan dan veteriner. 2001: 509–18.
- Haryuningtyas D. Deteksi mutasi pada gen tubulin β isotipe-1 cacing Haemonchus contortus isolat resisten terhadap benzimidazole dengan single strand conformation polymorphism. Jurnal Ilmu Ternak dan Veteriner (JITV). 2005;10(3):200–7.
- Kwa MS, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in β-tubulin isotype 1. Mol Biochem Parasitolol. 1994;63(2):299–303.
- Wiria AE, Hamid F, Wammes LJ, Kaisar MM, May L, Prasetyani MA, et al. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: a household-based cluster-randomized, double-blind, placebo-controlled trial. PLOS One. 2013;8(3):e57899.
- DNeasy® blood and tissue handbook. [internet] 2016 [diunduh pada 2016 01 20]. Available at: http:// diagnostics1.com/MANUAL/General_Qiagen.pdf.
- Hansen TV, Thamsborg SM, Olsen A, Prichard RK, Nejsum P. Genetic variations in the beta-tubulin gene and the internal transcribed spacer 2 region of Trichuris species from man and baboons. Parasit Vectors. 2013;6:236.
- DNA protocols and applications. [internet] 2016 [downloaded on 2016 01 20]. Available: https:// www.qiagen.com/de/resources/molecular-biologymethods/ dna/#Sample storage prior to extraction of genomic DNA.
- 260/280 and 260/230 ratios. [internet] 2016 [downloaded on 2016 06 09]. Available at: http:// www.nanodrop.com/Library/T009-NanoDrop%20 1000-&-NanoDrop%208000-Nucleic-Acid-Purity- Ratios.pdf.
Copyright @ 2018 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id