
Section Abstract Introduction Methods Results Discussion Conflict Of Interest Acknowledgment References
Clinical Research
Cryptosporidium spp. and rotavirus gastroenteritis and change of incidence after rotavirus vaccination among children in Raparin Pediatrics Hospital, Erbil, Iraq
pISSN: 0853-1773 • eISSN: 2252-8083
http://dx.doi.org/10.13181/mji.v26i3.1957 Med J Indones. 2017;26:190–7
Received: April 20, 2017
Accepted: August 10, 2017
Author affiliation:
1 Department of Medical Diagnostic Laboratory, Raparin Pediatrics Hospital, Erbil, Iraq
2 Department of Microbiology, College of Medicine, Hawler Medical University, Erbil, Iraq
Corresponding author:
Hadi M. Alsakee
E-mail: hadi.alsakee@med.hmu.edu.iq
Background
Watery diarrhea is the most common medical problem among infants and young children, caused by different microbial etiology including Cryptosporidium spp. and rotavirus, which are usually misdiagnosed in conventional stool test. This study aimed to investigate the incidence of Cryptosporidium and rotavirus gastroenteritis among children in Erbil as well as evaluate the efficacy of rotavirus vaccination procedure applied in Erbil.
Methods
Fecal specimens were collected from 400 children (boys and girls), aged one month to five years old, who attended Raparin Pediatrics Hospital in Erbil complaining from diarrhea, between January to August 2014. Modified Ziehl Neelsen technique and nested PCR were used for detection of cryptosporidiosis while rotavirus infection was detected by rapid CerTest.
Results
Rate of detection of cryptosporidiosis was remarkably higher using PCR than Ziehl-Neelsen stain (0% versus 6%), and the infection was slightly higher among boys (6.25% vs 5.55%) and children ≤2 years (11.7%). The peak of infection reached during spring season (March and April) (9.5%). The detection rate of rotavirus was 32.0%, which was slightly higher among males (34.4% vs 30.0%) and in children between one to three years old (39.3%). The highest detection rate (38.6%) was recorded during winter season (January and February). The infection was significantly higher among nonvaccinated children (65.9% vs 14.1%; p<0.05).
Conclusion
The incidence of cryptosporidiosis is declining. However, rotavirus gastroenteritis was relatively high among young children in Erbil. Rotateq vaccine significantly reduced the incidence of rotavirus infection.
Keywords
cryptosporidiosis, gastroenteritis, rotavirus, vaccine
Acute gastroenteritis or diarrhea is defined as the excretion of liquid stools three or more times a day. According to studies conducted by World Health Organization (WHO), diarrhea is estimated to be the second highest cause of morbidity and mortality among children in developing countries,1 for which there are various etiological agents in endemic areas such as bacteria, viruses, and parasites. Cryptosporidiosis is an enteric parasitic infection affecting a wide range of hosts, including humans, domestic and wild animals. It is also the most common cause of waterborne disease around the world.2
Successful management and prevention of this emerging disease requires knowledge of the diversity of species causing human disease and their zoonotic source. It commonly appears as intestinal infection in acquired immunedeficiency syndrome (AIDS) patients causing fatal diarrheal illness. It may also infect biliary system and respiratory tract. Children younger than five years of age are more commonly infected mainly in developing countries due to their immunological immaturity and their unhygienic behavior.3 Epidemiological studies reported that cryptosporidiosis is more prevalent in developing countries (5%–10%) than in developed countries (1%– 3%).4–6 Cryptosporidium spp. oocysts contain sporozoites which are the infectious forms excreted in feces of infected host. Humans acquire infections through direct contact with infected persons (anthroponotic transmission); animals (zoonotic transmission) or through ingestion of contaminated food or water.3
Rotaviruses are the most common cause of viral gastroenteritis in newborns and young children both in developing and developed countries. Studies have reported that each year rotavirus causes about 111 million episodes of diarrhea requiring only home care, two million hospitalizations and 400,000 deaths in children under five years of age; 82% of which occurs in children in the poorest countries.7 In Iraq, the mortality rate in children younger than five years of age was reported to be 130 per 1,000 for boys and 120 per 1,000 for girls in 2003.8 In Erbil governorate, very little information concerning rotavirus infection and Cryptosporidium gastroenteritis is available. Both infections cause profuse watery diarrhea in young children and are usually misdiagnosed because they cannot be detected by conventional stool test.
In the current study, we intended to investigate the incidence of rotavirus and Cryptosporidium gastroenteritis among children in Erbil in relation to demographic parameters and seasonal distribution as well as to evaluate the efficacy of rotavirus vaccine and vaccination procedure applied in Erbil.
METHODS
Design, setting, and duration of the study
This descriptive, cross sectional study was conducted in Erbil (capital city of Iraqi Kurdistan Region) with a population of around 1,370,000 inhabitants. The study population included 400 children complaining from sever watery diarrhea. The patients were from both genders, with ages ranging from one month to five years old. Patients (who passed watery or loose stools >3 times per day for >3 days) attending Raparin Pediatric Hospital in Erbil from January to August 2014, were included in this study.
This health service provider is a teaching hospital serves a population of ≈1.7 million (including refugees who dislocated from other Iraqi provinces). A careful history was obtained via a direct interview with the parents in which, basic demographic, epidemiologic, clinical information, and vaccination status were collected and stated in a closed-ended questionnaire sheet designed for such purpose. This project has been approved by the ethics committee in the College of Medicine, Hawler Medical University, and informed consent has already been obtained from their parents.
Collection and processing of fecal specimens
Fecal specimens were collected from the patients in clean, sterile, tightly covered wide mouth, disposable plastic containers. Each container was labeled with a number, date, and name of each subject. Furthermore, each fecal sample was divided into two parts by a clean and sterile wooden application. One used directly for macroscopic examination, direct wet mount preparation, modified Ziehl- Neelson technique (for detection of excreted Cryptosporidium spp. oocysts) and certest for detection of rotavirus infection whereas the second sample was kept frozen for molecular detection of cryptosporidiosis. Modified Ziehl- Neelsen staining technique was performed in accordance of Casemore.9 The CerTest rotavirus (CerTest biotec, Spain) was used for qualitative detection of rotavirus in stool samples. This test is one step colored chromatographic immunoassay. Rotavirus was detected in the fecal specimens following the instructions provided by the manufacturer. Fifty fecal samples that revealed negative tests for rotavirus and for cryptosporidiosis by modified Ziehl-Neelsen technique were selected to be tested by polymerase chain reaction (PCR).
Molecular detection of cryptosporidiosis Extraction of DNA
Deoxyribonucleic acid (DNA) was extracted from the fecal specimens using QIAamp DNA Stool Mini Kit (Qiagen, Germany). The extraction procedure was performed following the protocol provided by the manufacturer.
Polymerase chain reaction (PCR)
Nested PCR was performed to amplify 18S ribosomal ribonucleic acid (RNA) gene as described elsewhere.10 Primary amplification was carried out using the primers 5’-TTCTAGAGCTAATACATGCG 3’(F), and 5’CCCTAATCCTTCGAAACAGGA-3’ (R). The amplification products was then subjected to second PCR using the primers 5’-GGAAGGGTTGTATTTATTAGATAAAG-3’ (F), and 5’-AAGGAGTAAGGAACAACCTCCA-3’ (R). In both PCR rounds, the master mix involved 1.5 μL of primers, 1.5 μL of DNA sample and 47 μL of PCR reaction mixture (taqDNA polymerase, PCR buffer, dNTPs, gel loading dyes and novel green). The amplification was performed using PCR Thermal cycler (Eppendrof master cycle, France). Amplification protocol was consisted of 40 cycles of initial denaturation 30 seconds at 98°C, denaturation 10 seconds at 98°C, annealing 30 seconds at 55°C, extension 30 seconds at 72°C, and final extension 10 minutes at 72°C. Nested PCR amplification process consisted of 45 cycles of initial denaturation 30 seconds at 98°C, denaturation 10 seconds at 98°C, annealing 30 seconds at 61.4°C, extension 30 seconds at 72°C, and final extension 10 minutes at 72°C. The amplification products were detected on 1.5% agarose gel electrophoresis.
Statistical analysis
The collected data were statistically analyzed using statistical package for social sciences (SPSS) version 17 software. The association of two or more categorical variables was assessed by Chisquare test. Count and percentages were used to describe the frequency of different variables between all possible paired combinations of study groups. p≤0.05 was considered statistically significant.
RESULTS
Out of 400 stool samples that were collected from children who experienced gastroenteritis, all showed negative results for cryptosporidiosis by modified Ziehl-Neelsen technique. However, three samples (6%) out of fifty that were tested by PCR targeting 18 S rRNA gene, revealed positive bands at 550 bp (Figure 1). On the other hand, rotavirus infection was found to be positive in 128 (32.0%) of 400 cases. The incidence of cryptosporidiosis was almost similar among children who were from urban and rural areas. In contrast, rotavirus infection was significantly (p<0.05) higher among children who were from urban.
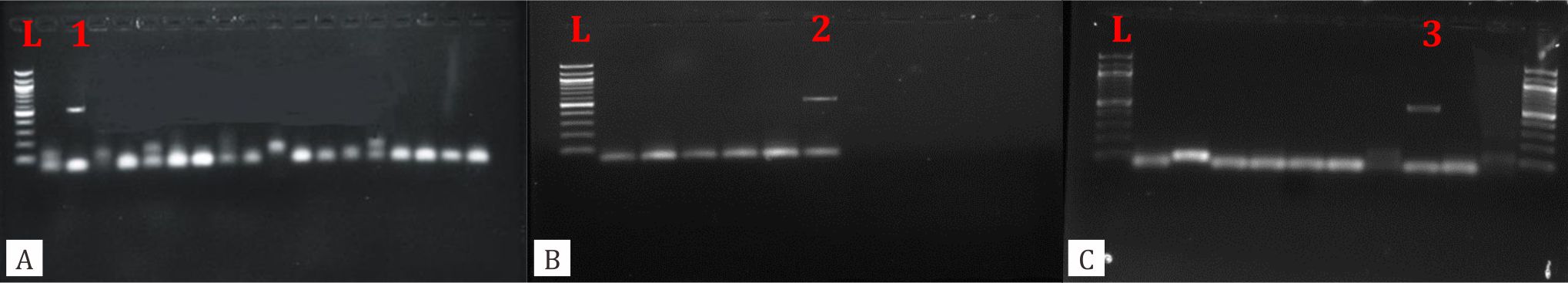
Figure 1. A, B, C, show the results of nested second round PCR using outer primers 5'-TTCTAGAGCTAATACATGCG 3'(F), 5'CCCTAATCCTTCGAAACAGGA- 3' (R) and inner primers 5'-GGAAGGGTTGTATTTATTAGATAAAG - 3' (F), and 5'-AAGGAGTAAGGAACAACCTCCA- 3' (R).Three positive bands (1, 2, 3) were detected at 550 bp region. L: 100 bp DNA ladder
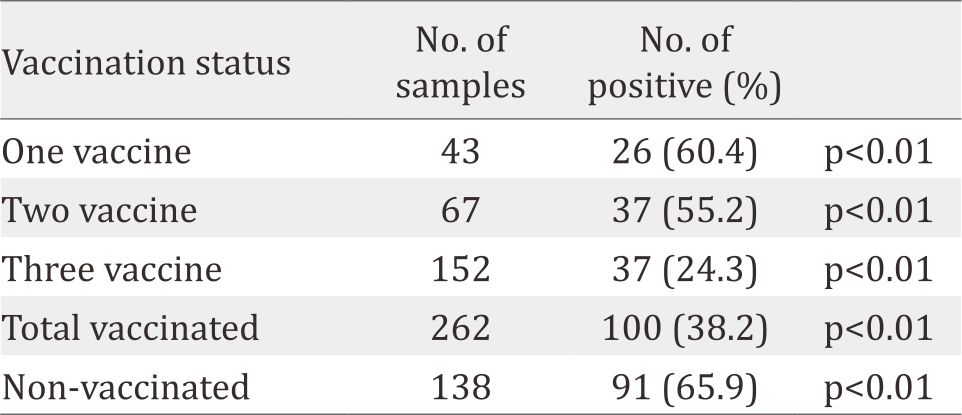
The frequency of infection for cryptosporidiosis and rotavirus infection among infected children was higher among males (6.25%, 34.4%, respectively) than females (5.55%, 30.0%, respectively) (Table 2). Cryptosporidiosis was higher through March and April (9.52%). In contrast, rotavirus infection was significantly (p<0.01) higher (38.6%) during winter months (January and February). The highest rate of cryptosporidiosis was observed in the cases with ages less than two years old (11.7%). However, rotavirus infection was significantly (p<0.05) higher among children younger than three years old. Table 3 indicates that rotavirus infection was significantly higher among non-vaccinated children, and the infection was sharply reduced among children who accomplished their vaccination protocol.
Table 1. Frequency of cryptosporidiosis and rotavirus infection according to residency
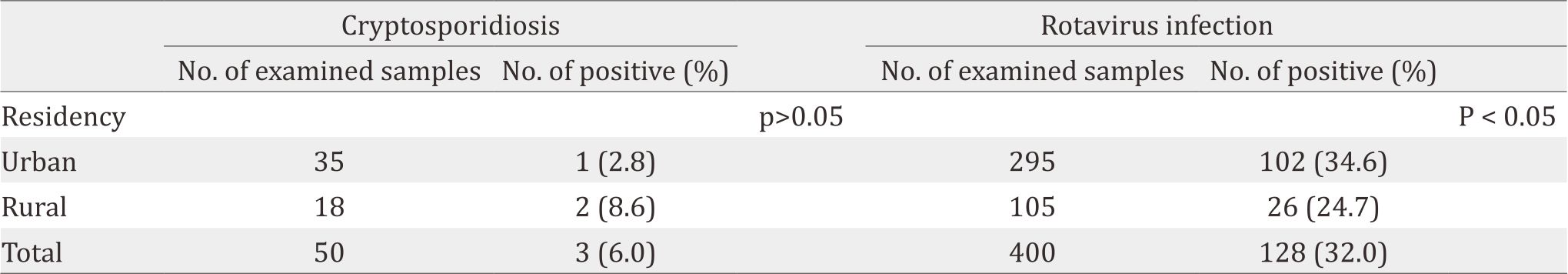
Table 2. Gender, age and seasonal distribution of cryptosporidiosis and rotavirus infection
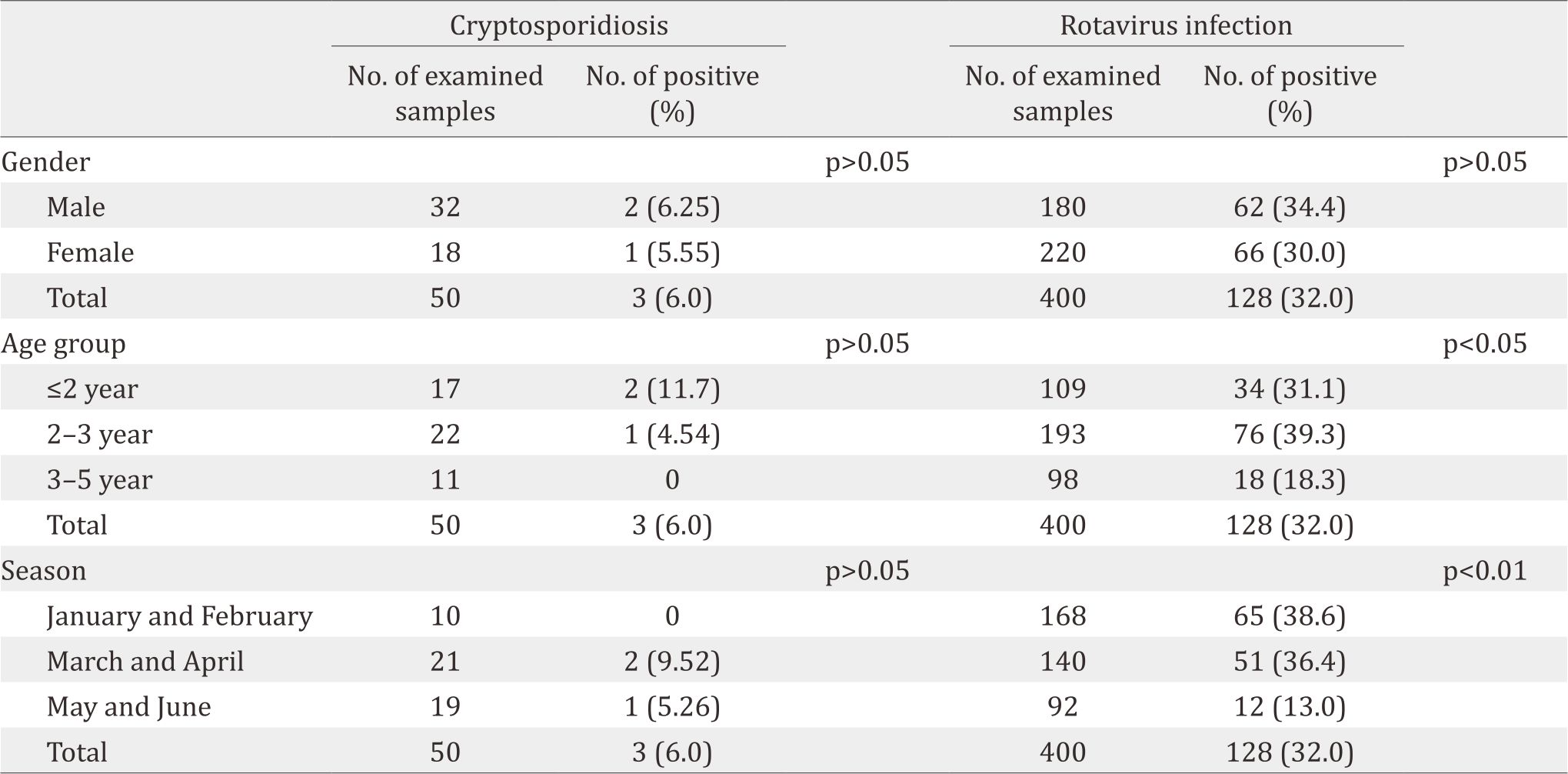
Table 3. Incidence change of rotavirus Gastroenteritis among 400 children in response to vaccination
DISCUSSION
Diarrheal illnesses in children are the major public health concern in developing countries, including Iraq as well as neighboring countries.4,11–14 According to WHO, diarrhea is regarded as the second highest cause of morbidity and mortality among the children in developing countries,1 for which cryptosporidiosis and rotavirus are the most common parasitic and viral cause for gastroenteritis worldwide.2 Comparing with studies carried out in other Iraqi provinces, our record was much lower than that reported by 11,12 in children aged less than three years old in Erbil and Sulaimani, respectively. However this finding was higher than that reported in some surrounding countries, Iran (4.6%)13 and Kuwait (3.4%).14 This variance of cryptosporidiosis might be attributed to some technical and epidemiological factors such as geographic location, drinking water facilities, diagnostic procedure, keeping and handling domesticated animals, particularly, cows, as well as other risk factors that could be associated with the acquisition of the infection.6
Superior of nested PCR in the diagnosis of cryptosporidiosis and false negative results of modified Ziehl-Neelson technique might be due to the scanty number of the oocysts that are excreted with the feces which could be under detectable level.10 Similar findings were observed by Maikai et al2 and Helmy et al10 Because of sensitivity and easily performance, PCR seems to be an obvious choice for detection of Cryptosporidium from the fecal specimens.10 The present study indicates that cryptosporidiosis is one of the causative agents of diarrhea, particularly among children, in Erbil along with other common parasitic agents such as Entamoeba histolytica and Giardia lamblia. Koyee et al11 found that cryptosporidiosis is the first common cause of diarrheal disease among children followed by amoebiasis.11 Although the rate of Cryptosporidium infection in this study was more prevalent among males than females, the differences was not significant. This finding was in agreement with previous studies conducted by other groups in Iraq.10,11
In this study, both genders were equally infected by Cryptosporidium oocysts. No known sex related physiological and epidemiological factors that changed the susceptibility and resistance to infection in certain gender. In contrast, a study performed by Saneian et al,13 found that cryptosporidiosis was more prevalent among females than males for which they could not refer to any factors as the causes of gender difference. Concerning the residency of the patients, the frequency of infection among rural dwellers was non-significantly higher than those in urban area. Interestingly our finding was in agreement with that observed by Koyee et al and Ali et al11,12 in Erbil and Sulaimani, at a national level and with that of Mahgoub et al15 in Jordan at international level, which could be explained by poor socioeconomic status, animal contact, and drinking non safe, and poor quality water. During the rainy season, heavy rains facilitate the spread of Cryptosporidium oocysts from infected animal feces, causing contamination of drinking water supplies. Therefore, the use of water purification systems and prohibition of grazing farm animals near the drinking water resources are important for preventing Cryptosporidium infection.6 For this reason, incidence pattern of cryptosporidiosis in livestock and other reservoirs should also be assessed in future studies in Erbil.
In the present study, the infection rate was found to be age-relevant, accordingly, since the infection rate was vary among the studied age groups. Most cases of cryptosporidiosis were detected among children within age group ≤2 years old. This finding was consistent with previously published data,3 which stated that cryptosporidiosis (43%) was much higher among children less than 4 years old in New South Wales, Australia, between January 2008 and December 2010. Consistently, Adamu et al. (2015),5 found that highest rate of cryptosporidiosis (46.8%) was among under two years old diarrheal children and who presented to a number of health care providers in Addis Ababa between January and March, 2004. Those authors attributed such age relevant infections to some personal and epidemiological practices such as contamination of milk bottles, creeping on a contaminated ground, and low personal hygiene as well as the lower levels of immunity against most infections including cryptosporidiosis. Therefore, a low infectious dose is sufficient to establish the infection, and since repeated exposure to the parasite could confer some sort of immunity against the infection, older children are more protected.16 Age was also found to be an important factor affecting the incidence of rotavirus gastroenteritis.17 We found that rotavirus infection was significantly (p<0.05) higher among children younger than 3 years of age comparing with children older than 3 years of age (70.4% vs 18.3%). Rotavirus infection can be occurred throughout the life, and symptomatic infection rates are the highest in children under two years of age and decrease progressively. The most severe symptoms tend to occur in children between six months to two years of age. This indicates that the first months of age are relatively protected from gastroenteritis, which might be due to transmitted maternal immunity through the placenta, lasting for the first few months of life. However, this immunity will be declined later on, increasing the risk of getting the infection through contaminated bottle milk feeding. The seasonal pattern of cryptosporidiosis showed that all cryptosporidiosis cases were detected over March, April, May, and June. This might be due to the relatively warm temperature and some other epidemiological factors in these months, such as little differences in the average of temperature and humidity as well as water abundance that facilitates contamination of drinking water supplies with animal feces and swage. On the other hand, the low percentage of infection in both winter months (January and February) might be due to the sever decrease in temperature in winter season which may reach 0°C or less, that has fatal impact on the oocysts viability and infectivity.18 Climate change is known to be another factor affecting the incidence of rotavirus infection. In studies done by other groups, they found high incidence of rotavirus gastroenteritis between December to April.19 Confirming our result, we found that peak incidence was in January and February (38.6%) followed by March and April (36.4%), and significantly lower incidence of infection was detected in May and June (13%) (p<0.05). Peak incidence during winter and spring seasons is possibly due to the moist and relatively cold period which might support viral stability in such climate.19 Since the samples were collected in the winter and spring seasons, the seasonal patterns of these infection were still obscure. Thus, further studies including summer season is recommended to provide a clear figure about seasonal distribution of cryptosporidiosis and rotavirus infection in Erbil.
Viruses are the most common causative agent for gastroenteritis which are estimated to cause about 70% of episodes of infectious diarrhea in children.20 Among viral gastroenteritis, rotaviruses are the most common causative agent in newborns and young children both in developing and developed countries, in agreement with published data at national and international level.17,19 The present study shows that rotavirus is one of the common causative agents of diarrheal illness in children in Erbil city besides other common parasitic and bacterial infections. The overall rate of rotavirus infection among studied children was 32%. Not much known concerning rotavirus infection in Erbil. A previous single study that had been carried out in Erbil city recorded that 37% of rotavirus infection was among children less than five years old.17 On national level, our data (32%) were close to it, as well as to that obtained and recorded in Thi-Qar (39%),21 but surprisingly they were much lower than that recorded in Najaf (66.7%).22 Comparably, the results were also close to that reported in Jordan (33%)23 and Turkey (32.4%),19 the neighboring countries. The results also revealed that the incidence of rotavirus infection was significantly higher in urban (33.6%) areas compared to that observed among children from rural (24.7%) areas. This finding was agreed with that reported by Hasson.21 These regional differences that have been reported in respect to the residency of the patients might be due to differences in health care systems, people health education, and sanitary awareness.21
Rotavirus vaccine is an effective tool for preventing severe rotavirus gastroenteritis. Attempts to develop a vaccine against human rotavirus began in the early 1980s. Two bands of rotavirus vaccines, namely, Rotateq (with three live attenuated rotavirus vaccine doses) and Rotarix (with two live attenuated vaccine doses) have been licensed and introduced for clinical use in many countries.24 The current study revealed that vaccinating children with Rotateq at different times (2nd, 4th and 6th months of age), significantly (p<0.05) declined the infection rate (14.1% vs 65.9%), indicating that the vaccine and the vaccination protocol that were applied in our communities were effective against rotavirus infection. This finding is supported by several other studies that have been carried out in this regard.24 The possible protective mechanisms of rotavirus vaccination as revealed by studies in animals and humans, involved rotavirus specific IgA antibodies in the gut lumen and rotavirus specific B cells expressing α4β7 intestinal receptor are important for protective immunity. On the other hand, rotavirus specific maternal antibody may interfere with the efficacy of the vaccination in infants and young animals.25
The present study has some limitations that the chance of detecting more positive cryptosporidiosis cases might be increased by increasing the number of the samples that selected to be tested by PCR since PCR appeared more efficient technique than Modifies Ziehl- Neelson staining technique. Furthermore, the study was enrolled children who attended only one health service provider (Raparin Pediatrics Hospital) during only six months. Therefore, more studies are required to support a true figure of cryptosporidiosis and rotavirus gastroenteritis in Erbil governorate.
In conclusion, the incidence of cryptosporidiosis is declining. However, rotavirus gastroenteritis is relatively high among young children in Erbil, and rotateq vaccine significantly reduces the incidence of this later infection.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
We would like to acknowledge the physicians, laboratory, and inpatient section staff of Raparin Pediatrics Hospital in Erbil for their kind assistance to arrange a direct interview with the parents and also for their valued assistance in collecting fecal specimen. Grateful thanks to dean and laboratory staff in Medical Research Center/ Hawler Medical University for their kind assistance to host the laboratory and molecular investigations.
REFERENCES
- Nyarango RM, Aloo PA, Kabiru EW, Nyanchongi BO. The risk of pathogenic intestinal parasite infections in Kisii Municipality, Kenya. BMC Public Health. 2008;8:237.
- Maikai B V, Umoh JU, Lawal IA, Kudi AC, Ejembi CL, Xiao L. Molecular characterizations of Cryptosporidium, Giardia, and Enterocytozoon in humans in Kaduna State, Nigeria. Exp Parasitol. 2012;131(4):452–6.
- Waldron LS, Dimeski B, Beggs PJ, Ferrari BC, Power ML. Molecular epidemiology, spatiotemporal analysis, and ecology of sporadic human cryptosporidiosis in Australia. Appl Environ Microbiol. 2011;77(21):7757–65.
- Areeshi MY., Beeching NJ., Hart CA. Cryptosporidiosis in Saudi Arabia and neighboring countries. Ann Saudi Med. 2007;27(5):325–32.
- Adamu H, Endeshaw T, Teka T, Kifle A, Petros B. The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiop J Heal Dev. 2006;20(1):39–46.
- Sim S, Yu J, Lee Y, et al. Prevalence of Cryptosporidium Infection among Inhabitants of 2 Rural Areas in White Nile State, Sudan. 2015;53(6):745–7.
- Narci H, Ugur M, Kisinma A, Turan H. Age distribution and seasonal pattern of rotavirus infection in children under 5 years. Jundishapur J Microbiol. 2013;6(1):16–9.
- Stephenson J. Health in Iraq. Jama. 2007;297(19):2069.
- Casemore DP. Laboratory methods for diagnosing cryptosporidiosis. J Clin Pathol. 1991;44(6):445–51.
- Helmy YA, Krücken J, Nöckler K, von Samson- Himmelstjerna G, Zessin KH. Molecular epidemiology of Cryptosporidium in livestock animals and humans in the Ismailia province of Egypt. Vet Parasitol. 2013;193(1–3):15–24.
- Koyee QM, Faraj AM. Prevalence of Cryptosporidium Spp. With other intestinal microorganisms among regular visitors of raparin pediatric hospital in Erbil City- Kurdistan Region, Iraq. Zanco J Pure Appl Sci. 2015;27(4):57–64.
- Ali FM, Ali SAK. Cryptosporidosis in Sulaimini Pediatric Teaching Hospital and comparison of different diagnostic methods for its detection. Eur Sci J. 2013;9(36):454–64.
- Saneian H, Yaghini O, Yaghini A, Modarresi M-R, Soroshnia M. Infection rate of Cryptosporidium parvum among diarrheic children in Isfahan. Iran J Pediatr. 2010;20(3):343–7.
- Iqbal J, Khalid N, Hira PR. Cryptosporidiosis in Kuwaiti children: Association of clinical characteristics with Cryptosporidium species and subtypes. J Med Microbiol. 2011;60(5):647–52.
- Mahgoub ES, Almahbashi A, Abdulatif B. Cryptosporidiosis in children in a north Jordanian paediatric hospital. East Mediterr Heal J. 2004;10(4– 5):494–501.
- Tahira, F, Khan, HM, Shukla I, Fatima, S, Malik, MA , Shahid M. Prevalence of Cryptosporidium in children with diarrhoea in North Indian Tertiary Care Hospital. J Community Med Heal Educ. 2012;2(3):1–3.
- Ahmed HM, Coulter JBS, Nakagomi O, et al. Molecular characterization of rotavirus gastroenteritis strains, Iraqi Kurdistan. Emerg Infect Dis. 2006;12(5):824–6.
- Mor SM, Tzipori S. Cryptosporidiosis in children in Sub- Saharan Africa: a lingering challenge. Clin Infect Dis. 2008;47(7):915–21.
- Ceyhan M, Alhan E., Salman N., et al. Multicenter prospective study on the burden of rotavirus gastroenteritis in Turkey, 2005-2006: A hospital-based study. J Infect Dis. 2009;200(Suppl 1):S234–8.
- Elliott EJ, Dalby-Payne J. 2. Acute infectious diarrhoea and dehydration in children. Med J Aust. 2004;181(10):565–70.
- Hasson DAJ. Prevalence of rota virus infection among children with acute gastroenteritis in Thi-Qar governorate. Thi-Qar Med J. 2009;3(1):88–100.
- Yasir S. Diagnostic Study on NSP5 of human rotavirus in Najaf Governorate. Kufa J Nurs Sci. 2015;5(3):1–9.
- Youssef M, Shurman A, Bougnoux M, Rawashdeh M, Bretagne S, Strockbine N. Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. FEMS Immunol Med Microbiol. 2000;28(3):257–63.
- Dulgheroff ACB, Figueiredo EF, Moreira LP, Moura LM, Gouvêa VS, Domingues AL. Distribution of rotavirus genotypes after vaccine introduction in the Triângulo Mineiro region of Brazil: 4-Year follow-up study. J Clin Virol. 2012;55(1):67–71.
- Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis. 2011;203(2):188–95.
Copyright @ 2017 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id