
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Basic Medical Research
Overexpression of p53 in extra large (more than 10 cm) hepatocellular carcinoma
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v27i2.1980 Med J Indones. 2018;27:71–5
Received: May 5, 2017
Accepted: May 6, 2018
Author affiliation:
1 Department of Surgery, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
2 Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Corresponding author:
Toar J.M. Lalisang
E-mail: toarlalisang@gmail.com
Background
Extra large (>10 cm) hepatocellular carcinoma (HCC) managed in our center shows a specific characteristic in tropical regions. This type of HCC exhibits distinct p53 expression. This study aimed to determine the association between p53 expression and tumor size and behavior.
Methods
Subjects with HCC who underwent surgical resection in our hospital during 2012–2015 were enrolled in this study. Subject’s characteristics, tumor size, histopathology findings, and tumor behavior were variables observed. An immunohistochemical study on p53 expression was conducted to determine its association with those variables.
Results
This study involved 38 subjects with tumor size ranging from 3 cm to 25 cm in diameter and 20 subjects (52.8%) with tumor size ranging from 10 cm to 25 cm in diameter. Only 13 samples were evaluated for p53 expression. Five subjects with >10 cm (extra large) tumor showed highly/overexpressed p53 (intensity>50%), two subjects with strong p53 expression (intensity>5%–50%), and two subjects with weak expression. Three subjects with <10 cm (large) tumor showed strong expression of p53 (5%–9%) and a subject with 3 cm tumor showed weak p53 expression (<5%). Highly expressed p53 was found in patients with microvascular invasion, inflammatory response, mitosis, and necrosis.
Conclusion
Overexpression of p53 was associated with extra large and poorly differentiated HCC.
Keywords
HCC, p53, poorly differentiated tumor, tumor size
Management of hepatocellular carcinoma (HCC) is challenging because the nature of the disease remains unclear. Earlier studies focused on the etiology of cirrhotic and non-cirrhotic cases, histopathology, classification, staging,1 surgical and non-surgical treatment, prognosis, and tumor recurrence.2,3 However, these topics are mostly population-specific. The inherent differences in HCC characteristic exist between western and eastern populations.4 In line with the development of molecular and genomic technologies in the last decade, research on a tumor’s characteristics has changed. Apoptosis and autophagy are actually dysregulated, which explains the progressivity of tumor proliferation and differentiation.5–7 Tumor suppressor protein 53 (p53) has increased as well. This p53 or "the guardian of genome" is known as a tumor suppressor gene that controls cellular growth after deoxyribonucleic acid (DNA) damage through mechanisms involving growth arrest and apoptosis; such mechanisms trigger a variety of antiproliferative programs by activating or repressing key effector genes.5,8,9 Mutation of p53 found in HCC has been reported after 2013.10
Patients with HCC managed in Cipto Mangunkusumo General Hospital (CMGH), Jakarta, have different characteristics from patients in studies published abroad. In this hospital, an immense tumor size measuring >10 cm in diameter is quite common. This particular characteristic might be related to underlying disease/tumor behavior.11 In addition, these cases of extra large tumor are presented as advanced cases in which delay in management is a major problem. However, published data regarding these characteristics are lacking. Even though resection of large tumors larger than 10 cm has been reported, the correlation of such tumors with p53 expression remains unclear.12–14
This obscurity becomes an issue when determining whether p53, as a tumor suppressor gene, is involved in the control of tumor enlargement or growth rate. Thus, this study focused on p53 expression in patients with HCC who presented such characteristics and underwent surgical resection to analyze the correlation of p53 expression with extra large tumors. Results may emphasize the characteristics and prognostic value,15 which can influence a survival. Therefore, this study aimed to determine the characteristics of p53 expression in extra large HCC.
METHODS
A retrospective study conducted on subjects with HCC underwent surgical resection. Subject’s characteristic including demographics, tumor size, and histopathological findings were observed. Immunohistochemical expression of p53 describing tumor cell immunoreactivity was analyzed in histopathological specimens. We used p53 polymer protein (DO–7; NovocastraTM), which is a liquid culture supernatant containing mouse monoclonal antibody and sodium azide as a preservative with specificity to human p53 wildtype and mutant forms under denaturing and non-denaturing conditions as primary antibody. Antigen/antibody/universal immunoperoxidase polymer complex (Histofine® Nichirei Biosciences) was used as secondary antibody. Immunohistochemical p53 expression of breast cancer was employed as control.
The expression of p53 was investigated under light microscopy using a scoring system of 0 to 3+ in the p53-positive region. The scores were classified in accordance with those employed by Qin et al,16 scores that represented immunoreactivity of <10% (–) were classified as weak expression, those of 10%–30% (+) indicated moderate expression, those of 31%–50% (++) suggested strong expression, and those of >50% (+++) demonstrated overexpression (Figure 1). Mitosis, microvascular invasion, distribution of inflammatory cells, and necrotic area were also the objects of investigation (Figure 2). Data were descriptively analyzed. The study was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia, with number 327/H2.F1/ETIK/2016.
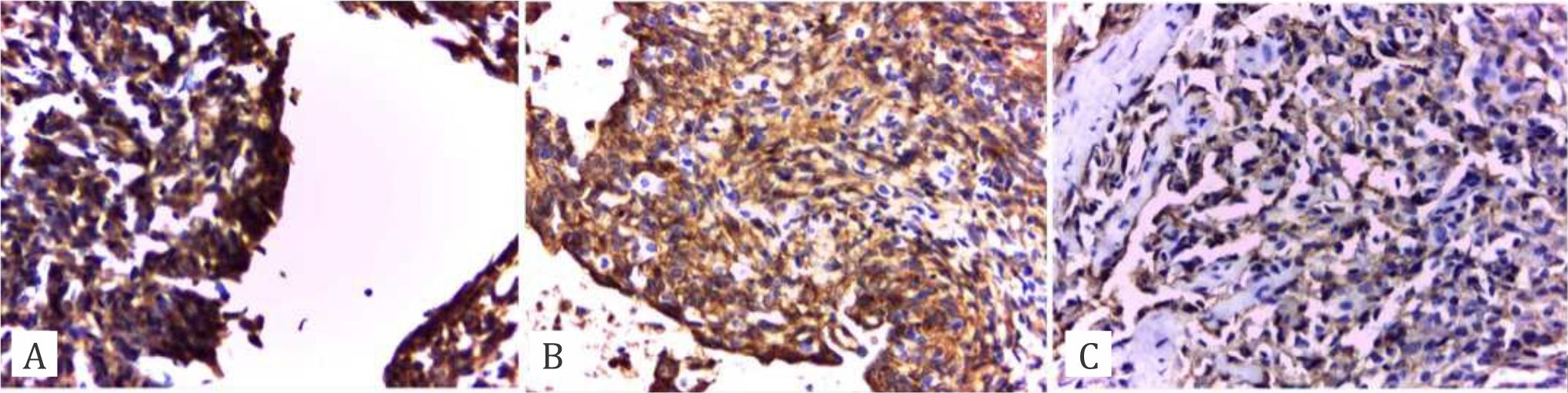
Figure 1. Immunohistochemical staining of p53 expression in HCC. A) Overexpression of p53 shows strong intensity of brown granules (>10%). B) Strong expression with moderate intensity (5%–9%). C) Low expression with intensity of <5%.
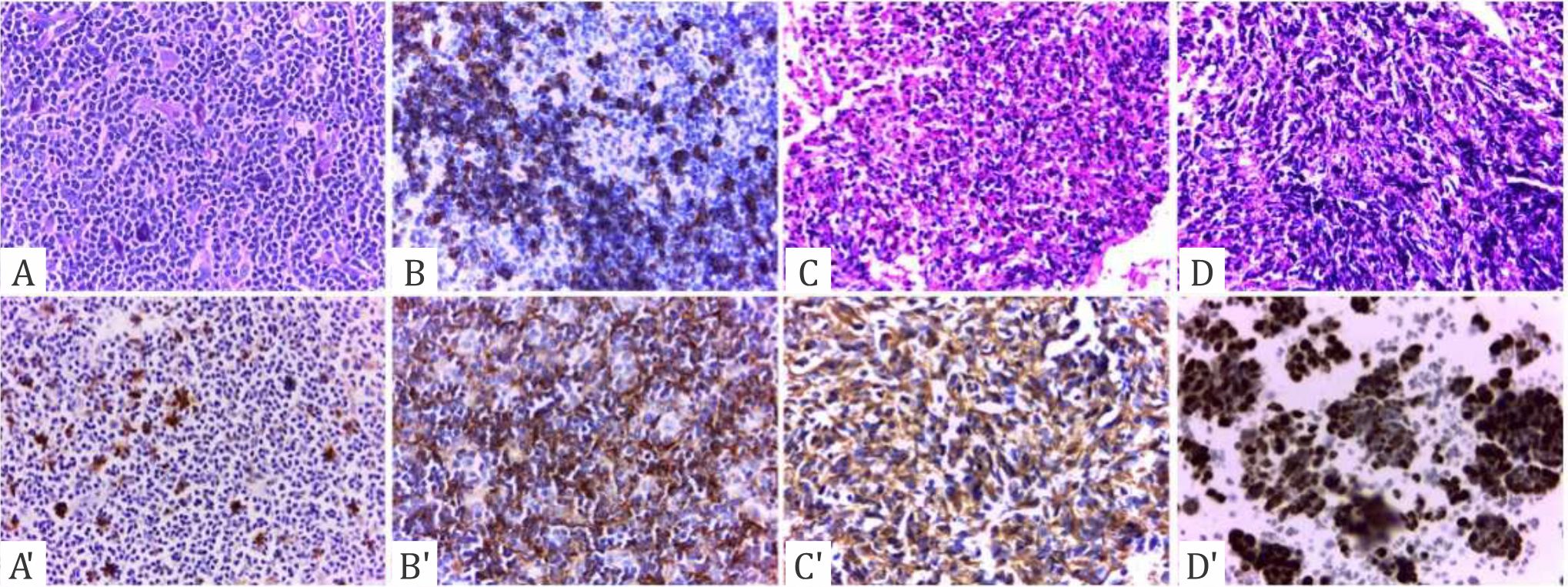
Figure 2. Immunohistochemical staining of p53 in HCC under light microscopy with objective lens magnification of 100 times. Upper row: A) Mitosis. B) Microvascular invasion. C) Inflammation. D) Necrosis. The distribution of highly absorbed IHCstained nuclei is shown. Lower row: Immunochemistry of p53 showing expression in A') Mitosis. B') Microvascular invasion. C') Inflammation. D') Necrosis. The expression of brown granules of p53 in high intensity (+++) is shown.
RESULTS
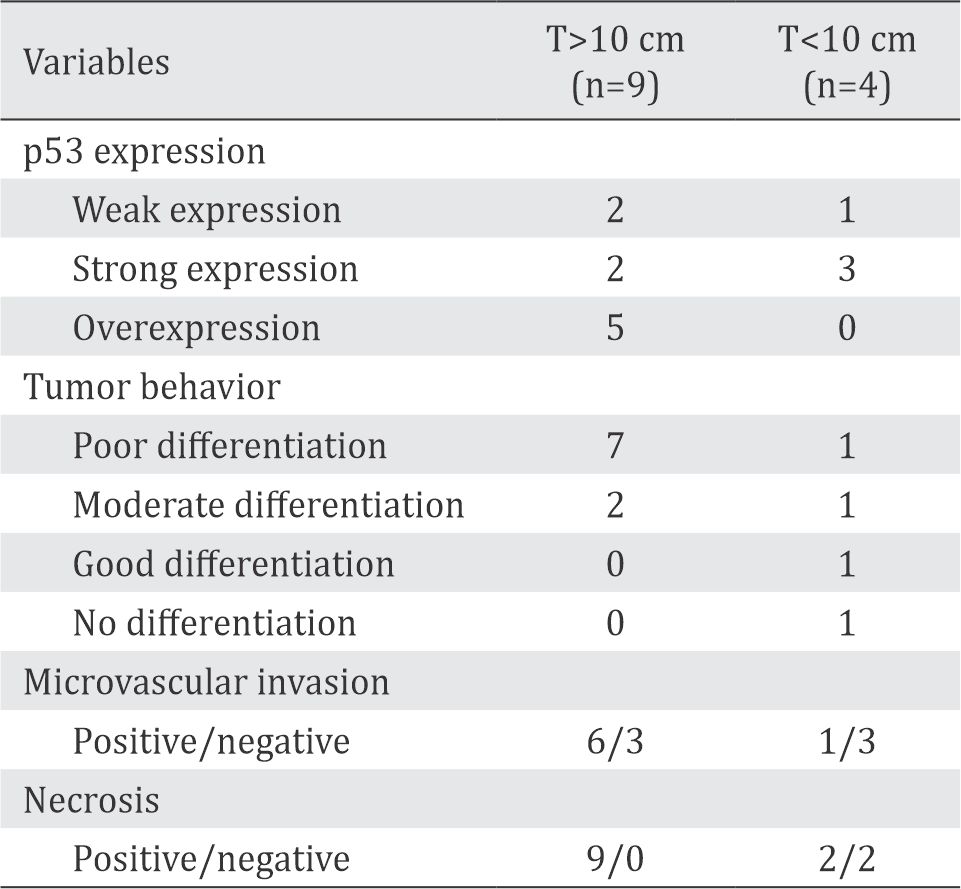
A total of 38 HCCs from 47 liver malignancies were managed in our hospital (CMGH) during the period of 2012–2015. These subjects were 17–78 years old with tumor size ranging from 3 cm to 25 cm in diameter, whereas 20 subjects (52.8%) were larger than 10 cm. Two subjects were recurrent, and the others were primary and had poor prognosis. Subject’s characteristics are shown in Table 1. p53 immunohistochemistry was obtained only from 13 samples. Eleven subjects (85%) showed increased p53 expression, with a tendency to increase with tumor size (Table 2).
Table 1. Subject's characteristics of HCC patients
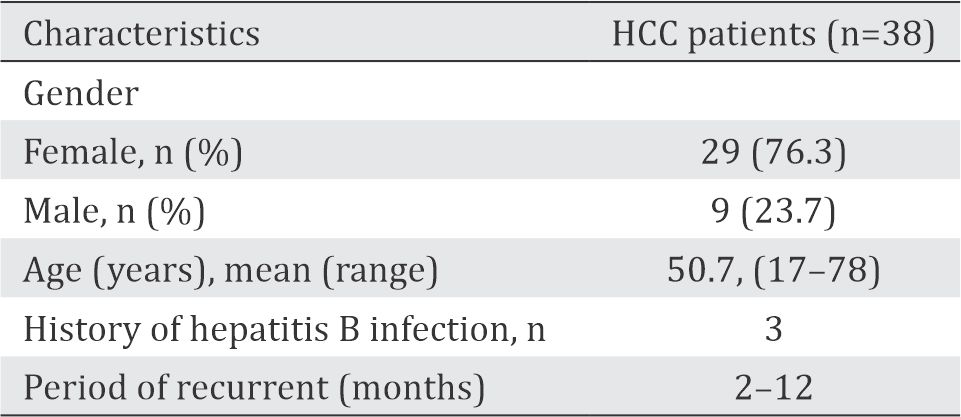
Table 2. Expression of p53, tumor size, and tumor behavior
DISCUSSION
This study investigated Indonesian characteristics of p53 expression in extra large HCCs managed in our hospital. Although the expression of large HCCs in Asia has previously been reported, the expression from Indonesia has a specific characteristic different from those previously reported.12–14 The extra large HCC diameter in our study was in the range of 10–25 cm, which differed from other reports.
This study found that p53 expression was consistent with tumor size depicting progressive tumor growth, aggressive proliferation, and differentiation of tumor cells. This increase in p53, known as the guardian of genome, expression demonstrated its protective and antiproliferative effects and tumor cell differentiation. The p53 tumor suppressor gene plays an important role to integrate multiple stress signals into a series of diverse antiproliferative responses. The most important role of p53 is to activate an apoptosis. Disrupted apoptosis may promote a tumor progression and chemoresistance. Apoptosis is apparently promoted by p53 through transcription via both dependent and independent mechanisms to ensure that apoptosis proceeds efficiently.17 Thus, elevated p53 in these extra large HCCs describing suppressor gene is required to control apoptosis. Overexpression suggests a provocative role.18 p53 gene represent different and biological characteristics at a time, thereby displaying a double-edged sword.19
However, the study of extra large (>10 cm) HCCs in our center demonstrated a tendency of p53 overexpression, which plays an important role in the progression of HCCs. The association of extra large HCCs with etiology remains unclear as the history of underlying disease was inadequately obtained; it might be related to mutant tumor suppressor gene of p53 found in characteristics of tumor biology due to specific etiology, which is viral hepatitis where the incidence of hepatitis in Indonesia is quite high.20–22 Mutant genes found in subjects with large and extra large tumors were in line with HCC behavior.9–11 Those with overexpressed p53 suggested poorly and moderately differentiated tumors, microvascular invasion, and necrotic area, thereby indicating poor prognosis.23 Among these behaviors, necrosis was predominantly found in this study, which suggested the nature of lesions present in surrounding tissues. Although the prevalence of necrosis was lower than those reported by Pedica et al23 (i.e. 30%), it was mostly found in poorly differentiated tumors.22,24 This characteristic was closely related to poor prognosis. Necrosis shown predominantly in these samples might be responsible for such an increase, portraying ongoing and prolonged oxidative stress leading to unrepaired DNA, which activates the pathway of this tumor suppressor gene.13,25
The limitations of this study were the sample size and emphasis on p53 immunohistochemistry. A total of 25 samples were unable to be processed possibly due to a tissue damage during the storage process. Samples obtained post-operatively for definitive diagnostic purposes cannot be used for further diagnostic plans, particularly for analyses on DNA/RNA and other proteins in the future. This scenario is one of the major obstacles in our institution. Thus, in 2014, our institution established Research Biobank to accommodate the need for high-quality tissue specimens and reliable data for research purposes.
This study investigated extra large tumors that depicted a different characteristic compared with those in previously reported work.1,2,4 Approximately 60.5% of our patients with extra large HCCs of >10 cm showed an increase in p53 expression, which was in line with a poor prognosis. Why the clinical presentation of these managed cases remains suitable for surgery and resection has yet to be elucidated. Size is one of the prediction indices for progressivity and procedure of resection. Thus, this study may be a basis for future work to gain insight into the continuity role of p53 in the progression of HCCs. In conclusion, overexpression of p53 was in line with extra large and poorly differentiated HCCs.
Conflicts of Interest
The authors affirm no conflict of interest.
Acknowledgment
This study funded by Grant of Universitas Indonesia 2015 No. 1570/UN2.RI12/ HKP.05.00/2015. A great appreciation is addressed to Rector of Universitas Indonesia, Director of Cipto Mangunkusumo General Hospital, and trainees who assist the study.
REFERENCES
- Ruiz I, Féray C. Current management of hepatocellular carcinoma. Cancer Radiother. 2015;19(6–7):410–5.
- Han JH, Kim DG, Na GH, Kim EY, Lee SH, Hong TH, et al. Evaluation of prognostic factors on recurrence after curative resections for hepatocellular carcinoma. World J Gastroenterol. 2014;20(45):17132–40.
- Truant S, Boleslawski E, Duhamel A, Bouras AF, Louvet A, Febvay C, et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. 2012;38(12):1189–96.
- Choo SP, Tan WL, Goh BK, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016;122(22):3430–46.
- Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15(5):513– 20.
- Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol. 2012;56(1):267–75.
- Zhou T, Ye L, Bai Y, Sun A, Cox B, Liu D, et al. Autophagy and apoptosis in hepatocellular carcinoma induced by EF25-(GSH)2: a novel curcumin analog. PLoS One. 2014;9(9):e107876.
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883.
- Kunst C, Haderer M, Heckel S, Schlosser S, Müller M. The p53 family in hepatocellular carcinoma. Transl Cancer Res. 2016;5(6):632–8.
- Muller PAJ, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25(3):304–17.
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Supplement 4):14–22.
- Liu J, Li W, Deng M, Liu D, Ma Q, Feng X. Immunohistochemical determination of p53 protein overexpression for predicting p53 gene mutations in hepatocellular carcinoma: a meta-analysis. PLoS One. 2016;11(7):e0159636.
- Yang XD, Pan LH, Wang L, Ke Y, Cao J, Yang C, et al. Systematic review of single large and/or multinodular hepatocellular carcinoma: surgical resection improves survival. Asian Pac J Cancer Prev. 2015;16(13):5541–7.
- Zhong JH, Rodríguez AC, Ke Y, Wang YY, Wang L, Li LQ. Hepatic resection as a safe and effective treatment for hepatocellular carcinoma involving a single large tumor, multiple tumors, or macrovascular invasion. Medicine. 2015;94(3):e396.
- Zhan P, Ji YN. Prognostic significance of TP53 expression for patients with hepatocellular carcinoma: a metaanalysis. Hepatobiliary Surg Nutr. 2014;3(1):11–7.
- Qin LX, Tang ZY, Ma ZC, Wu ZQ, Zhou XD, Ye QH, et al. P53 immunohistochemical scoring: an independent prognostic marker for patients after hepatocellular carcinoma resection. World J Gastroenterol. 2002;8(3):459–63.
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22(56):9030–40.
- Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V. P53 on the crossroad between regeneration and cancer. Cell Death Differ. 2017;24(1):8–14.
- Jiang P, Du W, Heese K, Wu M. The Bad guy cooperates with good cop p53: Bad is transcriptionally upregulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26(23):9071–82.
- Guan YS, He Q, La Z. Roles of p53 in carcinogenesis, diagnosis and treatment of hepatocellular carcinoma. J Cancer Mol. 2006;2(5):191–7.
- Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87(2):227–47.
- Graur F, Furcea L, Mois E, Biliuta A, Rozs AT, Negrean V, et al. Analysis of p53 protein expression in hepatocellular carcinoma. J Gastrointestin Liver Dis. 2016;25(3):345–9.
- Pedica F, Ruzzenente A, Bagante F, Capelli P, Cataldo I, Pedron S, et al. A re-emerging marker for prognosis in hepatocellular carcinoma: the add-value of FISHing c-myc gene for early relapse. PLoS One. 2013;8(7):e68203.
- Cioca A, Cimpean AM, Kundnani NR, Ceausu R, Suciu C, Raica M. P53 expression as a prognostic marker in hepatocellular carcinoma. Arch Biol Sci. 2014;66(2):841–5.
- Yang SF, Chang CW, Wei RJ, Shiue YL, Wang SN, Yeh YT. Involvement of DNA damage response pathways in hepatocellular carcinoma. Biomed Res Int. 2014;2014:153867.
Copyright @ 2018 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id