
Section Abstract Introduction Methods Results Discussion Conflict of Interest Acknowledgment References
Clinical Research
Poor treatment compliance leads to a higher mutation for rifampicin resistance in multibacillary leprosy patients
pISSN: 0853-1773 • eISSN: 2252-8083
https://doi.org/10.13181/mji.v27i4.1916 Med J Indones. 2018;27:237–43
Received: March 20, 2017
Accepted: August 30, 2018
Author affiliation:
Department of Dermatovenereology, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Corresponding author:
Yulia Siskawati
E-mail: yuliasiska@gmail.com
Background
Multidrug therapy (MDT) is a safe and effective drug combination for leprosy treatment that can prevent drug resistance. Mycobacterium leprae resistance, especially to rifampicin, is a serious problem as it potentially thwarts the worldwide leprosy-elimination program by the World Health Organization (WHO). One of the suspected causes of rifampicin resistance is poor treatment compliance. It was necessary to assess the association between the treatment compliance and the occurrence of mutation rifampicin resistance in multibacillary (MB) leprosy patients.
Methods
A comparative, analytical, cross-sectional study was performed in MB leprosy patients who had completed treatment at the Dermatovenereology Outpatient Clinic in Cipto Mangunkusumo Hospital and the Sitanala Center for Leprosy Hospital from October 2012 to April 2013. Based on treatment regularity and history of drug discontinuation, the subjects were classified as either having good or poor compliance. Skin smear from a slit skin smear (SSS) examination was further analyzed by using the polymerase chain reaction (PCR) sequencing technique to detect rifampicin resistance.
Results
Fifty-seven study subjects were enrolled in this study. In the good treatment compliance group (29 subjects), only 1 case of mutation for rifampicin resistance was found. Meanwhile, in the poor drug compliance group (28 subjects), 8 cases of mutation for resistance (29%) were found. This difference in mutation rate was statistically significant (OR=11.2; 95% CI=1.296–96.787; p=0.012).
Conclusion
This study revealed that the risk of occurrence of M. leprae resistance to rifampicin in patients with poor drug compliance was significantly higher than in those with good drug compliance.
Keywords
multibacillary leprosy, rifampicin resistance, treatment compliance
Leprosy is a chronic infectious disease caused by Mycobacterium leprae (M. leprae), which initially affects peripheral nerves and subsequently extends to nearby skin tissue, the oral mucosa, upper respiratory tract, reticuloendothelial system, eyes, muscles, bones, and testicles.1 Previously, dapsone was the only antibiotic given to treat leprosy. However, emerging resistance to this monotherapy strategy has led to the introduction of multidrug therapy (MDT) by the World Health Organization (WHO) in 1981.2–4 This MDT is a combination of drugs proven to be safe and effective to treat leprosy.5 For multibacillary (MB) leprosy, MDT consists of rifampicin, dapsone, and clofazimine, whereas for paucibacillary (PB) leprosy, it consists of rifampicin and dapsone.6 It is well known that administering 2 or more antibiotics with different mechanisms of action in combination can help to prevent drug resistance.3 Resistance to MDT, especially rifampicin, warrants special attention because it is a potential cause of failure of the worldwide leprosy-elimination program by the WHO.7
Rifampicin is a strong bactericidal antibiotic effective against both gram-positive and gram-negative bacteria.8 Single-dose of rifampicin is proven effective at eradicating 99.99% of M. leprae.9 Its bactericidal effectivity works through inhibition of DNA-dependent RNA polymerase, which is coded by the rpoB gene. Therefore, any mutation within the gene and/or its associated gene(s) could result in a conformational change in the polymerase that hinders rifampicin adherence.10 Another possible mechanism of rifampicin resistance is a change in bacteria cell wall permeability, which reduces the total amount of rifampicin available in the cell.8,10
Resistance to any component of leprosy MDT is confirmed by M. leprae inoculation on the sole of a guinea pig foot or by biomolecular study using polymerase chain reaction (PCR).11 This biomolecular study can detect specific drug-resistance-causing gene mutations.12 The mutation can be detected even if the specimen contains a low bacterial load.13
A study conducted by Maeda et al14 in 2001 encompassing several countries such as Japan, Haiti, Indonesia, Pakistan, and Philippines showed that 13 of 88 isolates (14.8%) are multidrug-resistant M. leprae. In 2007, Matsuoka11 found that from 252 isolates, rifampicin resistance in relapse cases reached 20% in Maluku Utara, Indonesia and Sulawesi Utara, Indonesia, while it was only 8.3% in Yangon, Myanmar. In this study, isolates were taken from all leprosy patients who fulfilled the inclusion criteria, regardless of completion of therapy.7
There are two possible etiologies for rifampicin resistance. Firstly, resistance is correlated with a previous history of rifampicin monotherapy.8,15 Secondly, it is related to poor compliance, including self-regulated drug administration and early treatment withdrawal.15 Several reasons underlying poor compliance are physical limitations (elderly and disability/ body deformity), inability to visit the physician during working hours, drug side effects, doubt in drug efficacy, social stigma, lack of reliable transportation, and location of the health care center far from home. However, Kar et al16 conducted a retrospective cohort study related to MDT compliance in 254 leprosy cases from 2002 to 2005 in India, and this study found that the poor compliance rate was higher in large urban centers, even though health care centers are readily available and the patients are much better educated than in rural areas.
This study was aimed to evaluate the association between rifampicin resistance and poor treatment compliance in MB leprosy patients after completion of treatment.
METHODS
This is an analytic, comparative, crosssectional study in MB leprosy patients who had completed a treatment course.
Subjects and sample size
Multibacillary leprosy patients at the Dermatovenereology Outpatient Clinic of the Cipto Mangunkusumo Hospital (PKK-RSCM) and Sitanala Center for Leprosy Hospital Tangerang with a positive slit skin smear (SSS) bacterial index who had completed an MDT treatment course were included in the study. Based on the previous study, the rifampicin-resistant mutation prevalence was 44% in the poor treatment compliance group and 14% in the good treatment compliance group.15 Therefore, the minimum number of subjects in each group using the sample size formula for proportion comparison was 28 subjects.
Study procedure
Patients who fulfilled the clinical eligibility criteria underwent SSS laboratory examination to determine if they met the positive bacterial index criteria. Three locations for SSS specimen collection were chosen according to previous SSS examination during the early evaluation. The blade which had been utilized to take a smear from each location was placed into an Eppendorf® tube for the M. leprae DNA suspension and extraction process using the QIAGEN QIAprep® Spin Miniprep Kit. Each specimen containing extracted M. leprae DNA underwent the next step, which was rpoB gene amplification, initially by PCR. Specimens showing negative results underwent subsequent nested PCR. Both PCR processes were carried out using the Takara® PCR Thermal Cycler (model TP 600). Finally, the amplified target gene was sequenced by using Dual CyCyeTM Terminator Sequencing Kits (ABI) to detect the specific nucleic acid arrangement.
Evaluation technique
Mutations in the rpoB gene that could cause significant resistance to rifampicin were defined as any type of mutation found in gene codon numbers 407, 416, 420, 425, and 427; or one amino acid insertion between codon numbers 408 and 409.17 At the same time, the subjects were classified into 2 groups based on the degree of treatment compliance, either good or poor. The group with poor treatment compliance included all subjects with a history of drug withdrawal for a period at certain point(s) during the MDT treatment course but in the end completed it, or irregular dapsone and clofazimine consumption defined as at least 7 days of not consuming both drugs in the same month.
Ethical considerations
This study passed ethical evaluation according to the standards of the Ethics Committee of the Faculty of Medicine, Universitas Indonesia and Research Division of Cipto Mangunkusumo Hospital (603/H2.F1/ETIK/2012). These standards were in accordance with those of the Helsinki declaration. All patients were agreed and gave informed consent for this study.
Data processing and analysis
All data were recorded and coded to be processed statistically using Statistical Product and Service Solutions (SPSS) 20.0 for Windows. The resistance rate was calculated by Chi-square test and presented as an odds ratio (OR) and 95% confidence interval (CI).
RESULTS
Subject characteristics
During a 7-month sampling period from October 2012 to April 2013, 57 patients were eligible for the study, 41 of which were from PKKRSCM and the rest were from Sitanala Center for Leprosy Hospital. Based on their compliance with treatment, there were 29 subjects with good compliance and 28 subjects with poor compliance. The baseline characteristics of these patients following classification into these groups is depicted in Table 1.
Table 1. Baseline characteristics of subjects
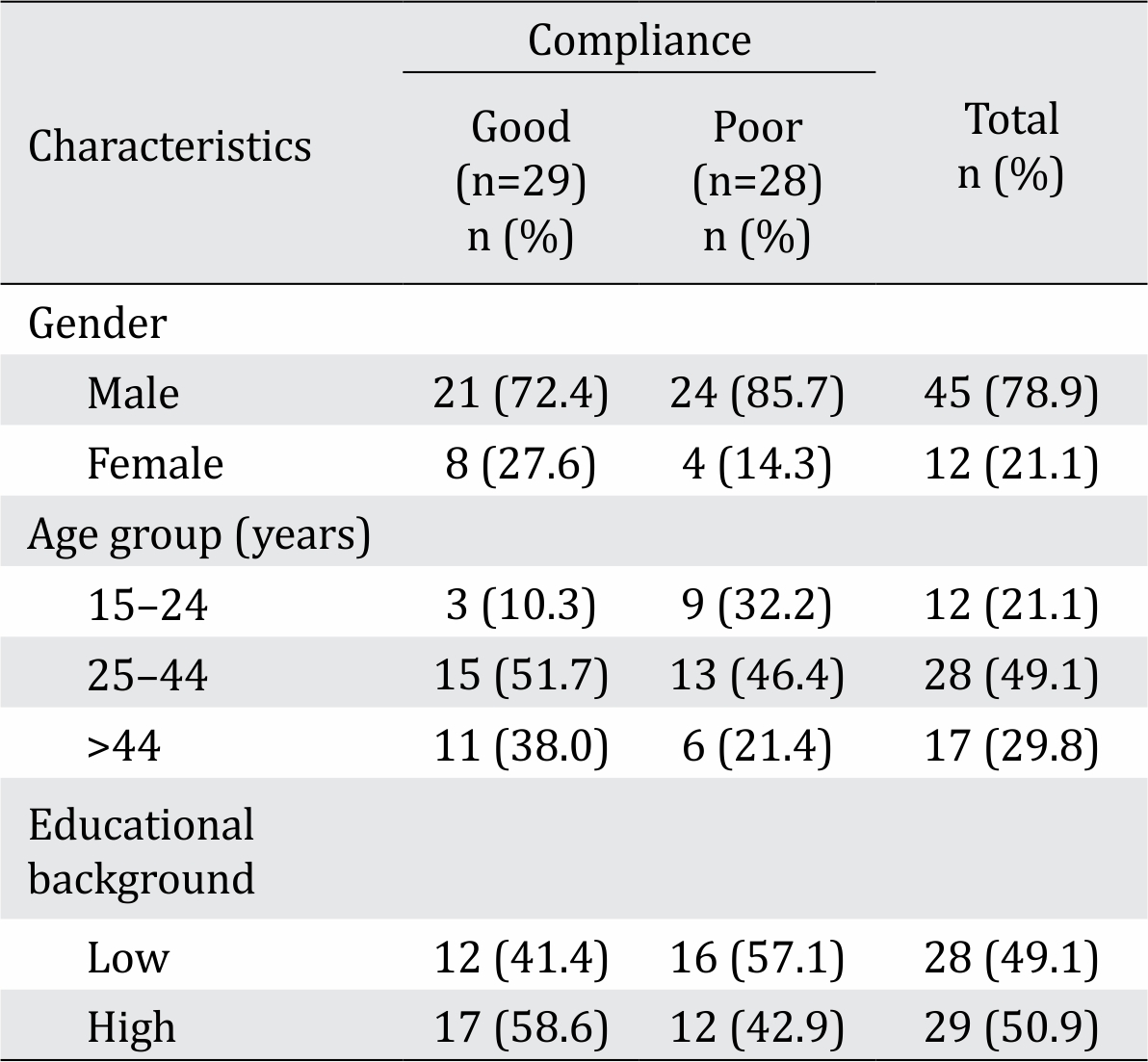
Several treatment history characteristics were evaluated. Regarding compliance, 97% of subjects with good compliance and 32% of those with poor compliance visited a healthcare center of at least once monthly. Specifically, monthly rifampicin consumption was adhered to in 100% and 79% of cases in the good and poor compliance groups, respectively. There was no history of rifampicin monotherapy in any subject from either group.
Clinically, 74% of all subjects were categorized as the “Borderline” (BL) type based on the Ridley & Jopling classification scheme. The distribution of each leprosy type by this classification between good and poor treatment compliance was almost identical.
PCR result and mutation pattern of the rpoB gene
Electrophoresis of the rpoB gene PCR product is shown in Figure 1. Of the 57 subjects, 48 were positive by PCR (PCR positivity rate 84%). All specimens with positive PCR results were subjected to the sequencing process to detect point mutations related to rifampicin resistance. Point mutations were detected in 9 samples (16%). The most common mutation found in 8 samples was at codon number 410, which encodes aspartic acid (GAT) in wild-type M. leprae but encodes tyrosine (TAT) in rifampicin-resistant mutant M. leprae, followed by mutation at codon number 425 (TCG encoding serine mutated into TTG encoding leucine) found in 1 sample (Figure 2). Additionally, there were 3 specimens with silent mutations: in 1 case, codon 420 CAC was mutated to CAT, both of which encode histidine, while in 2 other cases, codon 412 AAC was mutated to AAT, both of which encode asparagine.
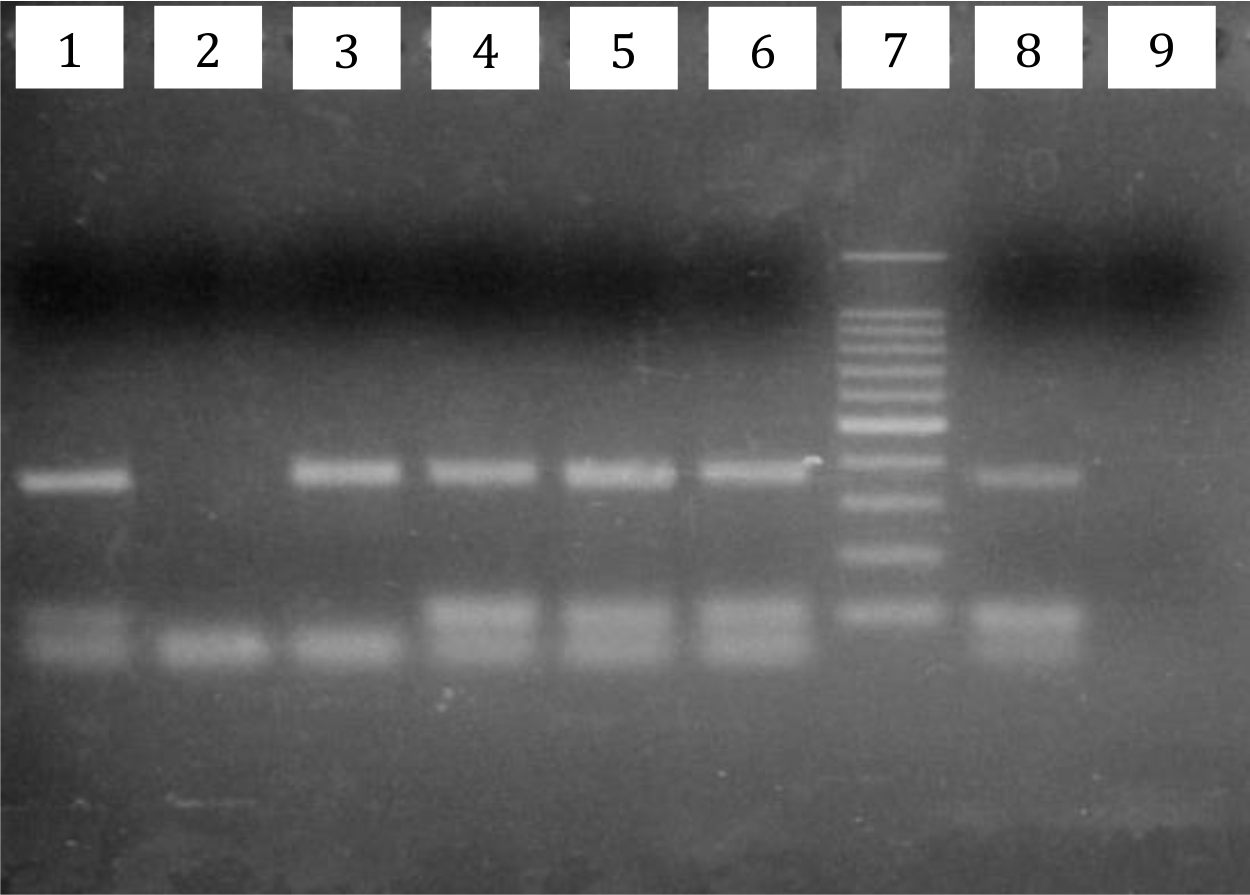
Figure 1. Electrophoresis result of M. leprae rpoB gene PCR from a slit skin smear (SSS) specimen. Wells number 1, 3, 4, 5, and 6, samples positive by PCR. Well number 7 was a 100- bp DNA ladder. Well number 8 was a positive control, and number 9 was a negative control
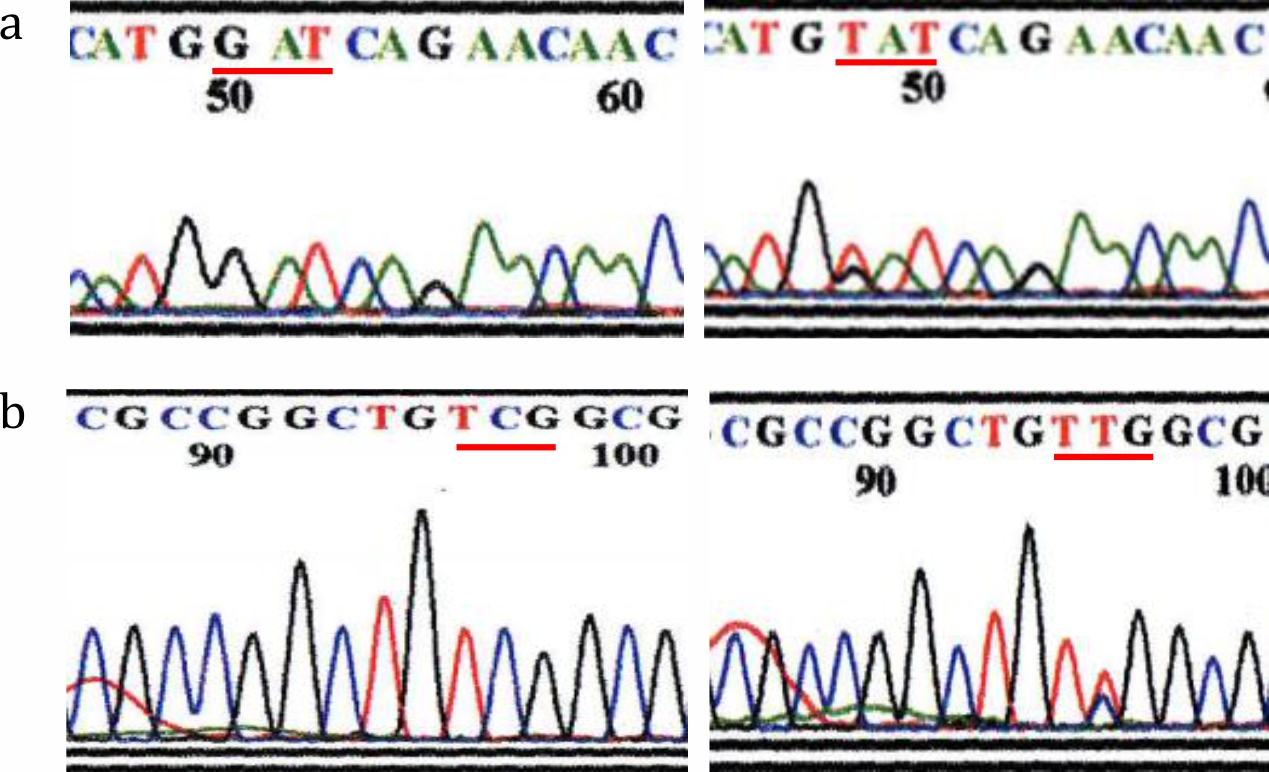
Figure 2. Nucleotide arrangement as a result of rpoB gene sequencing in: (a) codon number 410 showed a wild-type (GAT, left) and mutation (TAT, right); (b) codon number 425 showed a wild-type (TCG, left) and mutation (TTG, right)
Comparison of rifampicin resistance between patients with good and poor treatment compliance
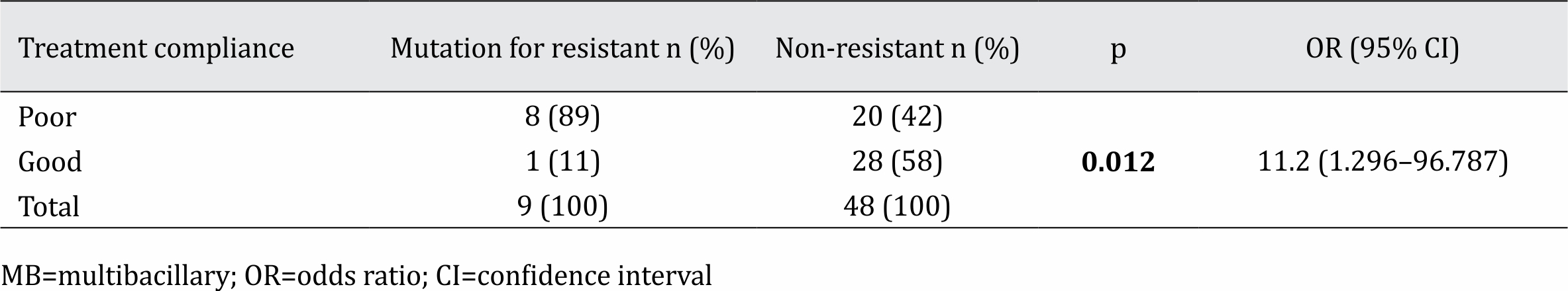
Out of 29 subjects with good treatment compliance, there were 25 samples (86%) positive by PCR, while positive PCR results were found in 23 (82%) out of 28 subjects with poor treatment compliance. Subjects with negative results were still included in the analysis and considered as not showing rifampicin resistance. When a sample is negative by PCR, it is assumed that there is no bacterial DNA detected in the sample, and no rifampicin resistance will be found. A statistical comparison of rifampicin resistance prevalence between the 2 groups is shown in Table 2.
Table 2. Comparison of rifampicin resistance based on treatment compliance in MB leprosy patients
DISCUSSION
Clinical characteristics of subjects
The ratio of male-to-female subjects was 3.75:1. These data were in agreement with a global epidemiology study which found a male bias in leprosy patients with the ratio of males to females ranging from 1.5 to 2:1.18,19 In addition, according to leprosy morbidity data at PKKRSCM in 2012, there were 152 (70.4%) and 64 (29.6%) new male and female leprosy patients, respectively. The male-to-female ratio reached 2.4:1.20 The majority (49.1%) of subjects were in the 25- to 44-year age group, the distribution of which was bimodal, one mode is the 35- to 44-year-old group.21 The previous PKK-RSCM leprosy data registry in 2012 showed an almost identical result: 49.2% of MB leprosy cases were in the 24- to 44 year-old age group.20 Lastly, in this study, nearly half of the subjects (49.1%) had a low educational level. After classification based on the degree of compliance, the difference in 1) male-to-female ratio, 2) age distribution, and 3) educational level between poor and good treatment compliance were not sufficient in magnitude to cause any significant bias in interpreting the analytical part of the study.
The MDT regimen for MB leprosy consisted of monthly (every 28 days) rifampicin and clofazimine (300 mg) that should be administered daily and under the supervision of a physician, self-administered dapsone and clofazimine (50 mg). Accordingly, the lower frequency of monthly visits to a healthcare center in those with poor compliance predicted lower monthly rifampicin consumption, one of the significant differences found in this study regarding treatment history characteristics. These data showed that the pre-determined criteria for the poor compliance group are aligned with rifampicin consumption regularity, which is presumably related to its resistance. Another major risk factor for rifampicin resistance is a history of rifampicin monotherapy, which was not found in any of the subjects included in this study. Therefore, history of rifampicin monotherapy could be excluded as the cause of resistance if any resistant isolate were to be found.
The dominance of the BL leprosy type in this study was commensurate with the PKKRSCM leprosy data registry in 2012, which found the BL type to be the most common (50.9%) among all new leprosy cases.20 Other than clinical manifestations, the leprosy classification by Ridley & Jopling also reflects the bacterial index.1 Supposedly, the almost identical distribution of leprosy types between good and poor treatment compliance would be followed by similar bacterial indices. Nevertheless, this study found a significant difference in bacterial index distribution between the 2 groups. The reason behind this finding was beyond the scope of this study and warrants further research. Fortunately, it did not appear to affect the PCR positivity rate, as this rate was similar in both groups.
In CRC patients, Campello et al18 designed a case-control study to investigate MCV in fresh tissue from 64 CRC patients and fresh biopsies from 80 matched relatives. They also examined 144 blood samples. After using PCR and sequencing, they reported that 6.3% of cases and 8.8% of controls were infected by MCV. Blood samples showed an overall rate of MCV infection of 12.5%. This result showed a low frequency of MCV in the colon, although they used fresh samples for analyses. The present study used FFPE samples, and we could not detect any MCV positive specimens. A low copy number of the MCV genome based on previous studies and using FFPE blocks could have affected our results. Also, due to the retrospective study design and not having any blood samples, we were not able to verify our results serologically. Our population had similar ages in the CRC group to those in the study by Campello et al,18 which showed that other confounding effects or different socioeconomic factors could produce negative results in the detection of MCV, although there was a detectable frequency of MCV in our country in different populations.9,17,28,29
Mutation pattern of rpoB gene
Not all specimens were positive by PCR. This finding could have resulted from fragmented M. leprae DNA that rendered it impossible to be detected through PCR, in which the primer used for rpoB gene consisting of 337–358 bp. Other possibilities were error in specimen handling during transportation from Jakarta to Surabaya and differences among laboratory workers’ experience and expertise.
The prevalence of significant mutations related to rifampicin resistance in this study was quite high, reaching 16%. Wahyuni et al22 reported only 1 case of rifampicin resistance among 270 isolates which were collected from 2003 to 2011. This prominent difference possibly occurred because of the difference in inclusion criteria; in this study, all subjects had completed the MDT course and had positive bacterial index results.
There were only 2 types of point mutation in this study: the most common being mutation of codon 410 GAT (aspartic acid) to TAT (tyrosine), followed by mutation of codon 425 TCG (serine) to TTG (leucine). A previous study by Matsuoka et al15 found that the most common mutation was codon 425 TCG (serine) to TTG (leucine), with a total number of 6 cases, followed by mutation in codon 410 GAT (aspartic acid) to TAT (tyrosine), codon 420 CAC (histidine) to GAC (aspartic acid), and codon 425 TCG (serine) to ATG (methionine), with 1 case each. Meanwhile, the only rifampicin-resistant case found in the study conducted by Wahyuni et al22 resulted from mutation of codon 410 GAT (aspartic acid) to TAT (tyrosine).
To date, there has not been a single report about the causal association between the point mutation found in this study and rifampicin resistance. To prove definitively whether the M. leprae isolates in the specimens were resistant to rifampicin, the gold standard diagnostic procedure was the inoculation of M. leprae on the foot of a guinea pig and observe its response to rifampicin treatment. Clinically, these leprosy patients whose isolates showed a point mutation need re-evaluation to detect a possible concurrent rifampicinresistance- related mutation. This suggestion was based on other studies in which the samples showed a point mutation in codon unrelated to rifampicin resistance. After re-evaluation, it was found that there was actually concomitant mutation of a codon related to rifampicin resistance.23,24
Association between treatment compliance and rifampicin resistance
There was a statistically significant relationship between treatment compliance and the occurrence of rifampicin resistance (p=0.012). MB leprosy patients whose treatment compliance was poor had a significantly higher risk of developing resistance to rifampicin than those whose compliance was good. A previous study by Matsuoka et al15 in 2007 reported that rifampicin resistance prevalence was as high as 20% in relapsing patients. One of the risk factors of leprosy relapse was poor compliance in previous treatment, and this study shows that high rifampicin resistance was related to treatment compliance. The rifampicin resistance rate in patients with poor compliance reached 89%, while it was only 11% in those with good treatment compliance.
In conclusion, poor treatment compliance was associated with a higher risk of rifampicin resistance in MB leprosy patients.
Conflicts of Interest
The authors affirm no conflict of interest in this study.
Acknowledgment
The authors would like to thank the Leprosy Laboratorium Tropical Disease Center Surabaya for the sample examination. We also express our sincere thanks to the Sitanala Hospital and its health branches for cooperation in providing the field of research.
REFERENCES
- Amirudin MD, Hakim Z, Darwis E. Diagnosis Penyakit Kusta. In: Sjamsoe-Daili ES, Menaldi SL, Ismiarto SP, Nilasari H, editors. Kusta. 1. 2 ed. Jakarta: Balai Penerbit FKUI; 2003. p. 12–32.
- Shepard CC, Rees RJ, Levy L, Pattyn SR, Baohong J, Dela Cruz EC. Susceptibility of strains of Mycobacterium leprae isolated prior to 1977 from patients with previously untreated lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1986;54(1):11–5.
- World Health Organization. WHO global strategy for further reducing the leprosy burden and sustaining leprosy control activities (Plan period: 2006-2010). Indian J Lepr. 2006;78(1):7–31.
- Guinto RS, Cellona RV, Fajardo TT, de la Cruz EC. Primary dapsone-resistant leprosy in Cebu, Philippines. Int J Lepr Other Mycobact Dis. 1981;49(4):427–30.
- Saonere JA. Leprosy: an overview. J Infect Dis Immun. 2011;3(14):233–43.
- Chemotherapy of leprosy. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;847:1–24.
- Drug resistance in leprosy: reports from selected endemic countries. Wkly Epidemiol Rec. 2009;84(26):264–7.
- Girdhar BK. Chemotherapy: Drugs Used in Leprosy Including Newer Drugs. In: Kar HK, Kumar B, editors. IAL Textbook of Leprosy. 1. 1 ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2010. p. 335–52.
- Ericsson CD, Steffen R, Ooi WW, Moschella SL. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32(6):930–7.
- Honore N, Cole ST. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993;37(3):414–8.
- Matsuoka M. Drug resistance in leprosy. Jpn J Infect Dis. 2010;63(1):1–7.
- Williams DL, Gillis TP. Molecular detection of drug resistance in Mycobacterium leprae. Lepr Rev. 2004;75(2):118–30.
- Saxena VM. In vitro methods for rapid monitoring of drug therapy and drug resistance in leprosy. Icmr Bull. 2001;31(8):1–6.
- Maeda S, Matsuoka M, Nakata N, Kai M, Maeda Y, Hashimoto K, et al. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob Agents Chemother. 2001;45(12):3635–9.
- Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz ED, et al. The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and relapsed leprosy patients from Myanmar, Indonesia and the Philippines. Lepr Rev. 2007;78(4):343–52.
- Kar S, Pal R, Bharati DR. Understanding non-compliance with WHO-multidrug therapy among leprosy patients in Assam, India. J Neurosci Rural Pract. 2010;1(1):9–13.
- Guidelines for global surveillance of drug resistance in leprosy. New Delhi: World Health Organization, Regional Office for South-East Asia, 2009.
- Williams DL, Waguespack C, Eisenach K, Crawford JT, Portaels F, Salfinger M, et al. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38(10):2380–6.
- Motta AC, Pereira KJ, Tarquínio DC, Vieira MB, Miyake K, Foss NT. Leprosy reactions: coinfections as a possible risk factor. Clinics (Sao Paulo). 2012;67(10):1145–8.
- Laporan data kunjungan pasien poliklinik Morbus Hansen rekapitulasi bulan Januari-Desember 2012. Jakarta: Departemen Ilmu Kesehatan Kulit dan Kelamin FKUI-RSCM, 2013. Indonesian.
- Brown TA. Mutation, repair, and recombination. Genomes. 1. 2 ed. Oxford: Wiley-Liss; 2002. p. 418–57.
- Wahyuni R, Adriaty D, Iswahyudi, Prakoeswa CR, Agusni I, Izumi S. Profile of mutation on drug resistance Mycobacterium leprae isolates in Indonesia collected during 2003–2011. Microbiol Indones. 2012;6(3):135–8.
- Honoré N, Roche PW, Grosset JH, Cole ST. A method for rapid detection of rifampicin-resistant isolates of Mycobacterium leprae. Lepr Rev. 2001;72(4):441–8.
- Cambau E, Bonnafous P, Perani E, Sougakoff W, Ji B, Jarlier V. Molecular detection of rifampin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin Infect Dis. 2002;34(1):39–45.
Copyright @ 2018 Authors. This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original author and source are properly cited.
mji.ui.ac.id